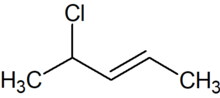
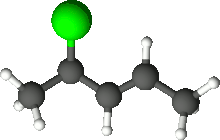
4-Chloro-2-pentene is an organic compound with the formula C5H9Cl. Its molecule is a linear chain of five carbon atoms, with a double bond between carbons 2 and 3 and a chlorine attached to carbon 4.
| |
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H9Cl | |
| Molar mass | 104.58 g·mol−1 |
| Density | 0.8988 g/cm3 at 20 °C[1] |
| Boiling point | 97 °C (207 °F; 370 K)[1] |
Refractive index (nD)
|
1.4322[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Physical and chemical properties
editAt room temperature, 4-chloro-2-pentene is a liquid with a density of ca. 0.9 g/cm3 and a boiling point of 97 °C.[1]
Synthesis
edit4-Chloro-2-pentene can be synthesized from its corresponding alcohol (3-pentene-2-ol) or from 1,3-pentadiene. In the latter case, 4-chloro-2-pentene can be obtained with a yield of 97%.[2]
Uses
edit4-Chloro-2-pentene has been used to prepare quaternary ammonium salts based on N,N,N',N'-tetramethyldiaminomethane, an intermediate in the manufacture of ionol (2,6-di-tert-butyl-4-methylphenol).[2][3]
4-Chloro-2-pentene readily reacts with stannyl lithium at low temperature to provide respective allyl stannanes.[4] Likewise, allyl silanes can be prepared from 4-chloro-2-pentene by silylation of the corresponding Grignard reagents with an appropriate chlorosilane.[5]
References
edit- ^ a b c d Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. p. 3.116. ISBN 9781498754293.
- ^ a b Levashova, V. I.; Nikonorova, N. I. (2009). "Synthesis and properties of quaternary ammonium salts based on N,N′-tetramethyldiaminomethane and 4-chloro-2-pentene". Petroleum Chemistry. 49 (3): 250–253. doi:10.1134/S0965544109030116. S2CID 94555175.
- ^ Rakhmatullin, R. R.; Levashova, V. I.; Dekhtyar', T. F. (2013). "Synthesis and properties of quaternary ammonium salts on the basis of piperidine". Petroleum Chemistry. 53 (2): 134–138. doi:10.1134/S0965544113020102. S2CID 96967040.
- ^ Carreira; Drabowicz; Fuerstner; Krause; Moloney; Carreira; Fuerstner; Molander; Thomas; Echavarren; Gouverneur; Hopkinson; Hou; Landelle; López-Carrillo; Łyz˙Wa; Paquin; Peng; Schatz; Seßler; Snaith; Wong; Yeung; Zhang, eds. (2011). "Product Subclass 28: Allylstannanes". Knowledge Updates 2011/2. doi:10.1055/sos-SD-105-00108. ISBN 9783131642813.
- ^ Fleming; Ley, eds. (2002). "Product Subclass 40: Allylsilanes". Category 1, Organometallics. doi:10.1055/sos-SD-004-00909. ISBN 9783131121714.