Vernolepin is a sesquiterpene lactone isolated from the dried fruit of Vernonia amygdalina. It shows platelet anti-aggregating properties[2] and is also an irreversible DNA polymerase inhibitor,[3] hence may have antitumor properties.
| |
| Names | |
|---|---|
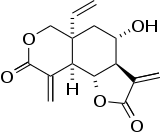
| Preferred IUPAC name
(3aR,4S,5aR,9aR,9bR)-5a-Ethenyl-4-hydroxy-3,9-dimethylideneoctahydro-2H-furo[2,3-f][2]benzopyran-2,8(3H)-dione | |
| Other names
Vernolepin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H16O5 | |
| Melting point | 179 to 180 °C (354 to 356 °F; 452 to 453 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
References
edit- ^ Laekeman, G. M.; Mertens, J.; Totté, J.; Bult, H.; Vlietinck, A. J.; Herman, A. G. (1 March 1983). "Isolation and Pharmacological Characterization of Vernolepin". Journal of Natural Products. 46 (2): 161–169. doi:10.1021/np50026a003. PMID 6410002.
- ^ Laekeman, G. M.; Clerck, F.; Vlietinck, A. J.; Herman, A. G. (1 January 1985). "Vernolepin: An antiplatelet compound of natural origin". Naunyn-Schmiedeberg's Archives of Pharmacology. 331 (1): 108–113. doi:10.1007/BF00498859. PMID 3934564. S2CID 10373287.
- ^ Clayden, Jonathan (2005). Organic chemistry (Reprinted (with corrections). ed.). Oxford [u.a.]: Oxford Univ. Press. pp. 238. ISBN 978-0-19-850346-0.