- For a less technical and generally accessible introduction to the topic, see Introduction to evolution.
| Part of the Biology series on |
| Evolution |
|---|
 |
| Mechanisms and processes |
| Research and history |
| Evolutionary biology fields |
The mechanisms and processes of evolutionary change includes selection (i.e. natural selection and artificial selection), polyploidy, hybridization, genetic drift, gene flow, and mutation. Natural selection favors genes that improve capacity for survival and reproduction. Genetic drift is random change in the frequency of alleles, caused by the random sampling of a generation's genes during reproduction. Gene flow is the transfer of genes within and between populations. Mutations are changes in the DNA sequence of a cell's genome.
The relative importance of natural selection and genetic drift in a population varies depending on the strength of the selection and the effective population size, which is the number of individuals capable of breeding.[1] Natural selection usually predominates in large populations, while genetic drift dominates in small populations. The dominance of genetic drift in small populations can even lead to the fixation of slightly deleterious mutations.[2] As a result, changing population size can dramatically influence the course of evolution. Population bottlenecks, where the population shrinks temporarily and therefore loses genetic variation, result in a more uniform population.[3] Bottlenecks also result from alterations in gene flow such as decreased migration, expansions into new habitats, or population subdivision.[1]
The outcome of the above mentioned mechanisms can be seen in form of adaptation, speciation and co-evolution. Adaptations are structures or behaviors that enhance a specific function, causing organisms to become better at surviving and reproducing, and speciation is the process where a species diverges into two or more descendant species. Speciation is the process where a species diverges into two or more descendant species and Co-evolution is the mutual evolutionary influence between two species.
Basic processes involved
editThe basic theory of evolution has three essential parts.
First, it is possible for the DNA of an organism to intermittently mutate. A mutation changes the DNA of an organism in a way that affects its offspring, either immediately or several generations down the line.[4]
Second, the change brought about by a mutation is either beneficial, harmful or neutral. A very small percentage of all mutations actually have a positive effect. These mutations lead to new versions of proteins that help an organism and its future generations better adapt to changes in their environment. For example, a specific 32 base pair deletion in human CCR5 (CCR5-Δ32) confers HIV resistance to homozygotes and delays AIDS onset in heterozygotes.[5] A neutral mutation is a mutation that occurs in an amino acid codon (presumably within an mRNA molecule) which results in the use of a different, but chemically similar, amino acid. Changes in DNA caused by mutation can cause errors in protein sequence, creating partially or completely non-functional proteins. To function correctly, each cell depends on thousands of proteins to function in the right places at the right times. When a mutation alters a protein that plays a critical role in the body, a medical condition can result. A condition caused by mutations in one or more genes is called a genetic disorder. However, only a small percentage of mutations cause genetic disorders; most have no impact on health. The process of selecting bad mutations and spreading good mutations is called natural selection.[4]
Third, as mutations occur and spread over long periods of time, they cause new species to form. Over the course of many millions of years, the processes of mutation and natural selection have created every biological individual that exists in the world today, from the simplest microorganisms such as bacteria and archaea, to multicellular organisms such as plants and animals.[4]
Before the process of evolution began, it is thought that billions of years ago, chemicals organized themselves into a self-replicating molecule.[4] This spark of life was the seed of every living thing that used to exist or which exists today. That simplest life form, through the processes of mutation and natural selection, was then shaped into every living species on the planet.
Genetic change in individuals
editMutation
editGenetic variation comes from random mutations that occur in the genomes of organisms. Mutations are changes in the DNA sequence of a cell's genome and are caused by radiation, viruses, transposons and mutagenic chemicals, as well as errors that occur during meiosis or DNA replication.[6][7][8] These mutagens produce several different types of change in DNA sequences; these can either have no effect, alter the product of a gene, or prevent the gene from functioning. Studies in the fly Drosophila melanogaster suggest that about 70 percent of mutations are deleterious, and the remainder are either neutral or have a weak beneficial effect.[9] Due to the damaging effects that mutations can have on cells, organisms have evolved mechanisms such as DNA repair to remove mutations.[6] Therefore, the optimal mutation rate for a species is a trade-off between short-term costs, such as the risk of cancer, and the long-term benefits of advantageous mutations.[10]
Large sections of DNA can also be duplicated, which is a major source of raw material for evolving new genes, with tens to hundreds of genes duplicated in animal genomes every million years.[11] Most genes belong to larger families of genes of shared ancestry.[12] Novel genes are produced either through duplication and mutation of an ancestral gene, or by recombining parts of different genes to form new combinations with new functions.[13][14] For example, the human eye uses four genes to make structures that sense light: three for color vision and one for night vision; all four arose from a single ancestral gene.[15] An advantage of duplicating a gene (or even an (entire genome) is that overlapping or redundant functions in multiple genes allows alleles to be retained that would otherwise be harmful, thus increasing genetic diversity.[16]
Changes in chromosome number may also involve the breakage and rearrangement of DNA within chromosomes. For example, two chromosomes in the Homo genus fused to produce human chromosome 2; this fusion did not occur in the chimpanzee lineage and chimpanzees retain these separate chromosomes.[17] In evolution, the most important role of such chromosomal rearrangements may be to accelerate the divergence of a population into new species by preserving genetic differences within populations.[18]
Sequences of DNA that can move about the genome, such as transposons, make up a major fraction of the genetic material of plants and animals, and may have been important in the evolution of genomes.[19] For example, more than a million copies of the Alu sequence are present in the human genome, and these sequences have now been recruited to perform functions such as regulating gene expression.[20] Another effect of these mobile DNA sequences is that when they move within a genome, they can mutate or delete existing genes and thereby produce genetic diversity.[21]
Polyploidy
editPolyploidy is the condition of some biological cells and organisms manifested by the presence of more than two homologous sets of chromosomes. Polyploid types are termed according to the number of chromosome sets in the nucleus: triploid (three sets; 3x), tetraploid (four sets; 4x), pentaploid (five sets; 5x), hexaploid (six sets; 6x) and so on.
A haploid has only one set of chromosomes. Haploidy may also occur as a normal stage in an organism's life cycle as in ferns and fungi. In some instances not all the chromosomes are duplicated and the condition is called aneuploidy. Where an organism is normally diploid, some spontaneous aberrations may occur which are usually caused by a hampered cell division.
Polyploidy occurs in some animals, such as goldfish, salmon, and salamanders, but is especially common among ferns and flowering plants, including both wild and cultivated species.[22] Wheat, for example, after millennia of hybridization and modification by humans, has strains that are diploid (two sets of chromosomes), tetraploid (four sets of chromosomes) with the common name of durum or macaroni wheat, and hexaploid (six sets of chromosomes) with the common name of bread wheat.[22] Many agriculturally important plants of the genus Brassica are also tetraploids; their relationship is described by the Triangle of U. The occurrence of polyploidy is a mechanism of speciation and is known to have resulted in new species of the plant Salsify (also known as "goatsbeard").
Examples in animals are more common in the ‘lower’ forms such as flatworms, leeches, and brine shrimp. Polyploid animals are often sterile, so they often reproduce by parthenogenesis. Polyploid salamanders and lizards are also quite common and parthenogenetic. While mammalian liver cells are polyploid, rare instances of polyploid mammals are known, but most often result in prenatal death.
There are large number of polyploid crop varieties. There are few naturally occurring polyploid conifers. One example is the giant tree Sequoia sempervirens or Coast Redwood which is a hexaploid (6x) with 66 chromosomes (2n=6x=66), although the origin is unclear [23]. The induction of polyploids is a common technique to overcome the sterility of a hybrid species during plant breeding. For example, Triticale is the hybrid of wheat (Triticum turgidum) and rye (Secale cereale). It combines sought-after characteristics of the parents, but the initial hybrids are sterile. After polyploidization, the hybrid becomes fertile and can thus be further propagated to become triticale. In some situations polyploid crops are preferred because they are sterile. For example many seedless fruit varieties are seedless as a result of polyploidy. Such crops are propagated using asexual techniques such as grafting.
Hybridization
editHybridization is the result of interbreeding between two animals or plants of different taxa. Hybridization between two closely related species is actually a common occurrence in nature. Many hybrid zones are known where the ranges of two species meet, and hybrids are continually produced in great numbers. These hybrid zones are useful as biological model systems for studying the mechanisms of speciation (Hybrid speciation). Recently DNA analysis of a bear shot by a hunter in the North West Territories confirmed the existence of naturally occurring and fertile polar bear-grizzly bear hybrids.[24]
In some species, hybridization plays an important role in evolutionary biology. While most hybrids are disadvantaged as a result of genetic incompatibility, the fittest survive, regardless of species boundaries. They may have a beneficial combination of traits allowing them to exploit new habitats or to succeed in a marginal habitat where the two parent species are disadvantaged. This has been seen in experiments on sunflower species. Unlike mutation, which affects only one gene, hybridization creates multiple variations across genes or gene combinations simultaneously. Successful hybrids could evolve into new species within 50-60 generations. This leads some scientists to speculate that life is a genetic continuum rather than a series of self-contained species.
Plant species hybridize more readily than animal species, and the resulting hybrids are more often fertile hybrids and may reproduce, though there still exist sterile hybrids and selective hybrid elimination where the offspring are less able to survive and are thus eliminated before they can reproduce.[25] A number of plant species are the result of hybridization and polyploidy with many plant species easily cross pollinating and producing viable seeds, the distinction between each species is often maintained by geographical isolation or differences in the flowering period. Animals, being more mobile, have developed complex mating behaviors that maintain the species boundary and when hybrids do occur, natural selection tends to weed them out of the population since these hybrids generally can not find mates that will accept them or they are less adapted and fit for survival in their habitats. Since plants hybridize frequently without much work, they are often created by humans in order to produce improved plants. These improvements can include the production of more or improved; seeds, fruits or other plant parts for consumption, or to make a plant more winter or heat hardy or improve its growth and/or appearance for use in horticulture. Much work is now being done with hybrids to produce more disease resistant plants for both agricultural and horticultural crops.[25] In many groups of plants hybridization has been used to produce larger and more showy flowers and new flower colors.
Genetic change in populations
editNatural selection
editNatural selection is the process by which genetic mutations that enhance reproduction become, and remain, more common in successive generations of a population. It has often been called a "self-evident" mechanism because it necessarily follows from three simple facts:
- Heritable variation exists within populations of organisms.
- Organisms produce more offspring than can survive.
- These offspring vary in their ability to survive and reproduce.
These conditions produce competition between organisms for survival and reproduction. Consequently, organisms with traits that give them an advantage over their competitors pass these advantageous traits on, while traits that do not confer an advantage are not passed on to the next generation.
The central concept of natural selection is the evolutionary fitness of an organism. This measures the organism's genetic contribution to the next generation. However, this is not the same as the total number of offspring: instead fitness measures the proportion of subsequent generations that carry an organism's genes.[26] Consequently, if an allele increases fitness more than the other alleles of that gene, then with each generation this allele will become more common within the population. These traits are said to be "selected for". Examples of traits that can increase fitness are enhanced survival, and increased fecundity. Conversely, the lower fitness caused by having a less beneficial or deleterious allele results in this allele becoming rarer—they are "selected against".[27] Importantly, the fitness of an allele is not a fixed characteristic, if the environment changes, previously neutral or harmful traits may become beneficial and previously beneficial traits become harmful.[28]
Natural selection within a population for a trait that can vary across a range of values, such as height, can be categorized into three different types. The first is directional selection, which is a shift in the average value of a trait over time—for example organisms slowly getting taller.[29] Secondly, disruptive selection is selection for extreme trait values and often results in two different values becoming most common, with selection against the average value. This would be when either short or tall organisms had an advantage, but not those of medium height. Finally, in stabilizing selection there is selection against extreme trait values on both ends, which causes a decrease in variance around the average value.[30] This would, for example, cause organisms to slowly become all the same height.
A special case of natural selection is sexual selection, which is selection for any trait that increases mating success by increasing the attractiveness of an organism to potential mates.[31] Traits that evolved through sexual selection are particularly prominent in males of some animal species, despite traits such as cumbersome antlers, mating calls or bright colors that attract predators, decreasing the survival of individual males.[32] This survival disadvantage is balanced by higher reproductive success in males that show these hard to fake, sexually selected traits.[33]
An active area of research is the unit of selection, with natural selection being proposed to work at the level of genes, cells, individual organisms, groups of organisms and even species.[34][35] None of these models are mutually-exclusive and selection may act on multiple levels simultaneously.[36] Below the level of the individual, genes called transposons try to copy themselves throughout the genome.[37] Selection at a level above the individual, such as group selection, may allow the evolution of co-operation, as discussed below.[38]
Artificial selection
editArtificial selection is the intentional breeding of certain traits, or combinations of traits, over others.[39] It was originally defined by Charles Darwin in contrast to the process of natural selection, in which the differential reproduction of organisms with certain traits is attributed to improved survival and reproductive ability in the natural habitat of the organism. Artificial selection that produces an undesirable outcome from a human perspective is sometimes called negative selection (but note that this term has a better-established meaning as a type of natural selection; see negative selection). Artificial selection can also be unintentional; it is thought that domestication of crops by early humans was largely unintentional.[40]
The difference between natural and artificial selection centers on the difference in environment among organisms subject to the two processes. Essentially, in artificial selection, the fitness, which is the amount of offspring an individual contributes to a population relative to other individuals in that same population of an organism, is defined in part by its display of the traits being selected for by human beings. Because humans either intentionally or unintentionally exert control over which organisms in a population reproduce or how many offspring they produce, the distribution of traits in the organisms' population will change.
It should be emphasized that there is no real difference in the genetic processes underlying artificial and natural selection, and that the concept of artificial selection was first introduced as an illustration of the wider process of natural selection. The selection process is termed "artificial" when human preferences or influences have a significant effect on the evolution of a particular population or species. Indeed, many evolutionary biologists view domestication as a type of natural selection and adaptive change that occurs as organisms are brought under the control of human beings.
Studies in evolutionary physiology, behavioral genetics, and other areas of organismal biology have also made use of deliberate artificial selection, though longer generation times and greater difficulty in breeding can make such projects challenging in vertebrates.[41][42]
Gene flow
editGene flow is the exchange of genes between populations, which are usually of the same species.[43] Examples of gene flow within a species include the migration and then breeding of organisms, or the exchange of pollen. Gene transfer between species includes the formation of hybrid organisms and horizontal gene transfer.
Migration into or out of a population can change allele frequencies. Immigration may add new genetic material to the established gene pool of a population. Conversely, emigration may remove genetic material. As barriers to reproduction between two diverging populations are required for the populations to become new species, gene flow may slow this process by spreading genetic differences between the populations. Gene flow is hindered by mountain ranges, oceans and deserts or even man-made structures such as the Great Wall of China, which has hindered the flow of plant genes.[44]
Horizontal gene transfer is the transfer of genetic material from one organism to another organism that is not its offspring, this is most common among bacteria.[45] In medicine, this contributes to the spread of antibiotic resistance, as when one bacterium acquires resistance genes it can rapidly transfer them to other species.[46] Horizontal transfer of genes from bacteria to eukaryotes such as the yeast Saccharomyces cerevisiae and the adzuki bean beetle Callosobruchus chinensis may also have occurred.[47][48] Viruses can also carry DNA between organisms, allowing transfer of genes even across biological domains.[49] Gene transfer has also occurred within eukaryotic cells, from the chloroplast and mitochondrial genomes to nuclear genomes.[50]
Genetic drift
editGenetic drift is the change in allele frequency from one generation to the next that occurs because alleles in the offspring generation are a random sample of those in the parent generation, and are thus subject to sampling error.[3] As a result, when selective forces are absent or relatively weak, allele frequencies tend to "drift" upward or downward in a random walk. This drift halts when an allele eventually becomes fixed, either by disappearing from the population, or replacing the other alleles entirely. Genetic drift may therefore eliminate some alleles from a population due to chance alone, and two separate populations that began with the same genetic structure can drift apart by random fluctuation into two divergent populations with different sets of alleles.[51] The time for an allele to become fixed by genetic drift depends on population size, with fixation occurring more rapidly in smaller populations.[52]
Although natural selection is responsible for adaptation, the relative importance of the two forces of natural selection and genetic drift in driving evolutionary change in general is an area of current research in evolutionary biology.[53] These investigations were prompted by the neutral theory of molecular evolution, which proposed that most evolutionary changes are the result the fixation of neutral mutations that do not have any immediate effects on the fitness of an organism.[54] Hence, in this model, most genetic changes in a population are the result of constant mutation pressure and genetic drift.[55]
Evolutionary processes
editAdaptation
editAdaptations are structures or behaviors that enhance a specific function, causing organisms to become better at surviving and reproducing.[56] They are produced by a combination of the continuous production of small, random changes in traits, followed by natural selection of the variants best-suited for their environment.[57] This process can cause either the gain of a new feature, or the loss of an ancestral feature. An example that shows both types of change is bacterial adaptation to antibiotic selection, with mutations causing antibiotic resistance by either modifying the target of the drug, or removing the transporters that allow the drug into the cell.[58] However, many traits that appear to be simple adaptations are in fact exaptations: structures originally adapted for one function, but which coincidentally became somewhat useful for some other function in the process.[59] One example is the African lizard Holapsis guentheri, which developed an extremely flat head for hiding in crevices, as can be seen by looking at its near relatives. However, in this species, the head has become so flattened that it assists in gliding from tree to tree—an exaptation.[59]
As adaptation occurs through the gradual modification of existing structures, structures with similar internal organization may have very different functions in related organisms. This is the result of a single ancestral structure being adapted to function in different ways. The bones within bat wings, for example, are structurally similar to both human hands and seal flippers, due to the common descent of these structures from an ancestor that also had five digits at the end of each forelimb. Other idiosyncratic anatomical features, such as bones in the wrist of the panda being formed into a false "thumb," indicate that an organism's evolutionary lineage can limit what adaptations are possible.[61]
During adaption, some structures may lose their original function and become vestigial structures.[62] Such structures may have little or no function in a current species, yet have a clear function in ancestral species, or other closely-related species. Examples include the non-functional remains of eyes in blind cave-dwelling fish,[63] wings in flightless birds,[64] and the presence of hip bones in whales and snakes.[65] Examples of vestigial structures in humans include wisdom teeth,[66] the coccyx,[62] and the vermiform appendix.[62]
An area of current investigation in evolutionary developmental biology is the developmental basis of adaptations and exaptations.[67] This research addresses the origin and evolution of embryonic development and how modifications of development and developmental processes produce novel features.[68] These studies have shown that evolution can alter development to create new structures, such as embryonic bone structures that develop into the jaw in other animals instead forming part of the middle ear in mammals.[69] It is also possible for structures that have been lost in evolution to reappear due to changes in developmental genes, such as a mutation in chickens causing embryos to grow teeth similar to those of crocodiles.[70]
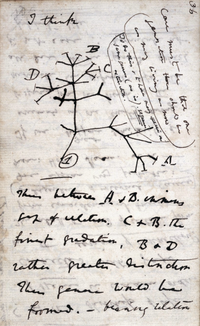
Speciation
editSpeciation is the process where a species diverges into two or more descendant species.[71] It has been observed multiple times under both controlled laboratory conditions and in nature.[72] In sexually-reproducing organisms, speciation results from reproductive isolation followed by genealogical divergence. There are four mechanisms for speciation. The most common in animals is allopatric speciation, which occurs in populations initially isolated geographically, such as by habitat fragmentation or migration. As selection and drift act independently in isolated populations, separation will eventually produce organisms that cannot interbreed.[73]
The second mechanism of speciation is peripatric speciation, which occurs when small populations of organisms become isolated in a new environment. This differs from allopatric speciation in that the isolated populations are numerically much smaller than the parental population. Here, the founder effect causes rapid speciation through both rapid genetic drift and selection on a small gene pool.[74]
The third mechanism of speciation is parapatric speciation. This is similar to peripatric speciation in that a small population enters a new habitat, but differs in that there is no physical separation between these two populations. Instead, speciation results from the evolution of mechanisms that reduce gene flow between the two populations.[71] Generally this occurs when there has been a drastic change in the environment within the parental species' habitat. One example is the grass Anthoxanthum odoratum, which can undergo parapatric speciation in response to localized metal pollution from mines.[75] Here, plants evolve that have resistance to high levels of metals in the soil. Selection against interbreeding with the metal-sensitive parental population produces a change in flowering time of the metal-resistant plants, causing reproductive isolation. Selection against hybrids between the two populations may cause reinforcement, which is the evolution of traits that promote mating within a species, as well as character displacement, which is when two species become more distinct in appearance.[76]
Finally, in sympatric speciation species diverge without geographic isolation or changes in habitat. This form is rare since even a small amount of gene flow may remove genetic differences between parts of a population.[77] Generally, sympatric speciation in animals requires the evolution of both genetic differences and non-random mating, to allow reproductive isolation to evolve.[78]
One type of sympatric speciation involves cross-breeding of two related species to produce a new hybrid species. This is not common in animals as animal hybrids are usually sterile, because during meiosis the homologous chromosomes from each parent, being from different species cannot successfully pair. It is more common in plants, however because plants often double their number of chromosomes, to form polyploids. This allows the chromosomes from each parental species to form a matching pair during meiosis, as each parent's chromosomes is represented by a pair already.[79]
Indeed, chromosome doubling can itself cause reproductive isolation, as half the doubled chromosomes will be unmatched when breeding with undoubled organisms.[80]
Speciation events are important in the theory of punctuated equilibrium, which accounts for the pattern in the fossil record of short "bursts" of evolution interspersed with relatively long periods of stasis, where species remain relatively unchanged.[81] In this theory, speciation and rapid evolution are linked, with natural selection and genetic drift acting most strongly on organisms undergoing speciation in novel habitats or small populations. As a result, the periods of stasis in the fossil record correspond to the parental population, and the organisms undergoing speciation and rapid evolution are found in small populations or geographically-restricted habitats, and therefore rarely being preserved as fossils.[82]
Co-evolution
editCo-evolution is the mutual evolutionary influence between two species. Each party in a co-evolutionary relationship exerts selective pressures on the other, thereby affecting each others' evolution.[83] Co-evolution includes the evolution of a host species and its parasites, and examples of mutualism evolving through time. Evolution in response to abiotic factors, such as climate change, is not coevolution (since climate is not alive and does not undergo biological evolution). Evolution in a one-on-one interaction, such as that between predator and prey, host-symbiont or host-parasite pair, is coevolution. But many cases are less clearcut: a species may evolve in response to a number of other species, each of which is also evolving in response to a set of species. This situation has been referred to as "diffuse coevolution". And, certainly, for many organisms, the biotic (living) environment is the most prominent selective pressure, resulting in evolutionary change.[83]
Co-evolution also occurs between predator and prey species as in the case of the Rough-skinned Newt (Taricha granulosa) and the common garter snake (Thamnophis sirtalis). In this case, the newts produce a potent nerve toxin that concentrates in their skin. Garter snakes have evolved resistance to this toxin through a set of genetic mutations, and prey upon the newts. The relationship between these animals has resulted in an evolutionary arms race that has driven toxin levels in the newt to extreme levels. (see Red Queen).[83]
Co-evolutionary algorithms are also a class of algorithms used for generating artificial life as well as for optimization, game learning and machine learning.[83] Pioneering results in the use of co-evolutionary methods were by Daniel Hillis (who co-evolved sorting networks) and Karl Sims (who co-evolved virtual creatures).
Macroevolution
editMacroevolution is a scale of analysis of evolution in separated gene pools.[84] Macroevolutionary studies focus on change that occurs at or above the level of species, in contrast with microevolution,[85] which refers to smaller evolutionary changes (typically described as changes in allele frequencies) within a species or population. The process of speciation may fall within the purview of either, depending on the forces thought to drive it. Paleontology, evolutionary developmental biology, comparative genomics and genomic phylostratigraphy contribute most of the evidence for the patterns and processes that can be classified as macroevolution. An example of macroevolution is the appearance of feathers during the evolution of birds from one group of dinosaurs.
Arguments over macroevolution in opposition to microevolution have waxed and waned through most of the 20th century. Primarily, paleontologists and other evolutionary biologists advanced a variety of non-Darwinian evolutionary processes as explanations for patterns found in the fossil record, emphasizing macroevolution as a source of morphologic innovation.[86] Later, paleontologists, from Simpson to Gould, Stanley, and others, accepted the primacy of natural selection but debated that rapid speciation produced a discontinuity between micro- and macroevolution.[86] This second segment emphasizes the sorting of innovations between species. Other discontinuities emerge in the persistence of trends (differential success of species within clades), including species sorting, in the differential success between clades and in the initiation and establishment of evolutionary novelties. These discontinuities inflict a hierarchical structure to evolution and disgraced any smooth extrapolation from allelic substitution to large-scale evolutionary patterns. Recent developments in comparative developmental biology recommend a need to re-evaluate the possibility that some macroevolutionary discontinuities may be associated with the origination of evolutionary innovation.[86] The attractiveness of macroevolution reflects the comprehensive documentation of large-scale patterns which expose a richness to evolution unexplained by microevolution.
See also
editFurther readings
edit- Gould, Stephen Jay (2002). The Structure of Evolutionary Theory. Cambridge: Belknap Press (Harvard University Press). ISBN 0-674-00613-5.
- Futuyma DJ (2005). Evolution. Sunderland: Sinauer Associates. ISBN 0-878-93187-2.
- Mayr E (2001). What Evolution Is. New York: Basic Books. ISBN 0-465-04426-3.
- Coyne JA, Orr HA (2004). Speciation. Sunderland: Sinauer Associates. ISBN 0-878-93089-2.
- Smith JM, Szathmáry E (1997). The Major Transitions in Evolution. Oxfordshire: Oxford University Press. ISBN 0-198-50294-X.
- Nicholas H. Barton, Derek E.G. Briggs, Jonathan A. Eisen, David B. Goldstein and Nipam H. Patel (2007). Evolution. Cold Spring Harbor Laboratory Press. ISBN 0-879-69684-2.
{{cite book}}: CS1 maint: multiple names: authors list (link)
References
edit- ^ a b Whitlock M (2003). "Fixation probability and time in subdivided populations". Genetics. 164 (2): 767–79. PMID 12807795.
- ^ Ohta T (2002). "Near-neutrality in evolution of genes and gene regulation". PNAS. 99 (25): 16134–37.
- ^ a b Harwood AJ (1998). "Factors affecting levels of genetic diversity in natural populations". Philos. Trans. R. Soc. Lond., B, Biol. Sci. 353 (1366): 177–86. PMID 9533122.
- ^ a b c d Basic processes of evolution HowStuffWorks, Inc. Retrieved on 10-16-2007
- ^ "CCR5 receptor gene and HIV infection, Antonio Pacheco".
- ^ a b Bertram J (2000). "The molecular biology of cancer". Mol. Aspects Med. 21 (6): 167–223. PMID 11173079.
- ^ Aminetzach YT, Macpherson JM, Petrov DA (2005). "Pesticide resistance via transposition-mediated adaptive gene truncation in Drosophila". Science. 309 (5735): 764–67. doi:10.1126/science.1112699. PMID 16051794.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Burrus V, Waldor M (2004). "Shaping bacterial genomes with integrative and conjugative elements". Res. Microbiol. 155 (5): 376–86. PMID 15207870.
- ^ Sawyer SA, Parsch J, Zhang Z, Hartl DL (2007). "Prevalence of positive selection among nearly neutral amino acid replacements in Drosophila". Proc. Natl. Acad. Sci. U.S.A. 104 (16): 6504–10. PMID 17409186.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sniegowski P, Gerrish P, Johnson T, Shaver A (2000). "The evolution of mutation rates: separating causes from consequences". Bioessays. 22 (12): 1057–66. PMID 11084621.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Carroll SB, Grenier J, Weatherbee SD (2005). From DNA to Diversity: Molecular Genetics and the Evolution of Animal Design. Second Edition. Oxford: Blackwell Publishing. ISBN 1-4051-1950-0.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Harrison P, Gerstein M (2002). "Studying genomes through the aeons: protein families, pseudogenes and proteome evolution". J Mol Biol. 318 (5): 1155–74. PMID 12083509.
- ^ Orengo CA, Thornton JM (2005). "Protein families and their evolution-a structural perspective". Annu. Rev. Biochem. 74: 867–900. PMID 15954844.
- ^ Pál C, Papp B, Lercher MJ (2006). "An integrated view of protein evolution". Nat. Rev. Genet. 7 (5): 337–48. PMID 16619049.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bowmaker JK (1998). "Evolution of colour vision in vertebrates". Eye (London, England). 12 (Pt 3b): 541–47. PMID 9775215.
- ^ Gregory TR, Hebert PD (1999). "The modulation of DNA content: proximate causes and ultimate consequences". Genome Res. 9 (4): 317–24. PMID 10207154.
- ^ Zhang J, Wang X, Podlaha O (2004). "Testing the chromosomal speciation hypothesis for humans and chimpanzees". Genome Res. 14 (5): 845–51. PMID 15123584.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ayala FJ, Coluzzi M (2005). "Chromosome speciation: humans, Drosophila, and mosquitoes". Proc. Natl. Acad. Sci. U.S.A. 102 Suppl 1: 6535–42. PMID 15851677.
- ^ Hurst GD, Werren JH (2001). "The role of selfish genetic elements in eukaryotic evolution". Nat. Rev. Genet. 2 (8): 597–606. PMID 11483984.
- ^ Häsler J, Strub K (2006). "Alu elements as regulators of gene expression". Nucleic Acids Res. 34 (19): 5491–97. PMID 17020921.
- ^ Aminetzach YT, Macpherson JM, Petrov DA (2005). "Pesticide resistance via transposition-mediated adaptive gene truncation in Drosophila". Science. 309 (5735): 764–67. PMID 16051794.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Tate, J.A., Soltis, D.E., & Soltis, P.S. (2005). Polyploidy in plants. In The Evolution of the Genome (edited by T.R. Gregory). Elsevier, San Diego, pp.371-426.
- ^ Ahuja MR, Neale DB. "Origins of Polyploidy in Coast Redwood (Sequoia sempervirens (D. DON) ENDL.) and Relationship of Coast Redwood to other Genera of Taxodiaceae" Silvae Genetica 51, 2–3 (2002)
- ^ "Hybrid bear shot dead in Canada". BBC News. 2006-05-13.
{{cite news}}: Check date values in:|date=(help) - ^ a b Wricke, Gunter, and Eberhard Weber. 1986. Quantitative genetics and selection in plant breeding. Berlin: W. de Gruyte
- ^ Haldane J (1959). "The theory of natural selection today". Nature. 183 (4663): 710–13. PMID 13644170.
- ^ Lande R, Arnold SJ (1983). "The measurement of selection on correlated characters". Evolution. 37: 1210–26}. doi:10.2307/2408842.
- ^ Futuyma, Douglas J. (2005). Evolution. Sunderland, Massachusetts: Sinauer Associates, Inc. ISBN 0-87893-187-2.
- ^ Hoekstra H, Hoekstra J, Berrigan D, Vignieri S, Hoang A, Hill C, Beerli P, Kingsolver J (2001). "Strength and tempo of directional selection in the wild". Proc. Natl. Acad. Sci. U.S.A. 98 (16): 9157–60. PMID 11470913.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Felsenstein (1979). "Excursions along the Interface between Disruptive and Stabilizing Selection". Genetics. 93 (3): 773–95. PMID 17248980.
- ^ Andersson M, Simmons L (2006). "Sexual selection and mate choice". Trends Ecol. Evol. (Amst.). 21 (6): 296–302. PMID 16769428.
- ^ Kokko H, Brooks R, McNamara J, Houston A (2002). "The sexual selection continuum". Proc. Biol. Sci. 269 (1498): 1331–40. PMID 12079655.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hunt J, Brooks R, Jennions M, Smith M, Bentsen C, Bussière L (2004). "High-quality male field crickets invest heavily in sexual display but die young". Nature. 432 (7020): 1024–27. PMID 15616562.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gould SJ (1998). "Gulliver's further travels: the necessity and difficulty of a hierarchical theory of selection". Philos. Trans. R. Soc. Lond., B, Biol. Sci. 353 (1366): 307–14. PMID 9533127.
- ^ Mayr E (1997). "The objects of selection". Proc. Natl. Acad. Sci. U.S.A. 94 (6): 2091–94. PMID 9122151.
- ^ Maynard Smith J (1998). "The units of selection". Novartis Found. Symp. 213: 203–11, discussion 211–17. PMID 9653725.
- ^ Hickey DA (1992). "Evolutionary dynamics of transposable elements in prokaryotes and eukaryotes". Genetica. 86 (1–3): 269–74. PMID 1334911.
- ^ Gould SJ, Lloyd EA (1999). "Individuality and adaptation across levels of selection: how shall we name and generalize the unit of Darwinism?". Proc. Natl. Acad. Sci. U.S.A. 96 (21): 11904–09. PMID 10518549.
- ^ Artificial selection Annenberg Media, December 2007
- ^ Artificial Selection powerpoint, University of Wisconsin-Madison
- ^ Swallow JG, Garland T. (2005). Selection experiments as a tool in evolutionary and comparative physiology: insights into complex traits—an introduction to the symposium. Integr Comp Biol, 45:387–390.
- ^ Garland T. (2003). Selection experiments: an under-utilized tool in biomechanics and organismal biology. Ch.3, Vertebrate Biomechanics and Evolution ed. Bels VL, Gasc JP, Casinos A.
- ^ Morjan C, Rieseberg L (2004). "How species evolve collectively: implications of gene flow and selection for the spread of advantageous alleles". Mol. Ecol. 13 (6): 1341–56. PMID 15140081.
- ^ Su H, Qu L, He K, Zhang Z, Wang J, Chen Z, Gu H (2003). "The Great Wall of China: a physical barrier to gene flow?". Heredity. 90 (3): 212–19. PMID 12634804.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Boucher Y, Douady CJ, Papke RT, Walsh DA, Boudreau ME, Nesbo CL, Case RJ, Doolittle WF (2003). "Lateral gene transfer and the origins of prokaryotic groups". Annu Rev Genet. 37: 283–328. PMID 14616063.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Walsh T (2006). "Combinatorial genetic evolution of multiresistance". Curr. Opin. Microbiol. 9 (5): 476–82. PMID 16942901.
- ^ Kondo N, Nikoh N, Ijichi N, Shimada M, Fukatsu T (2002). "Genome fragment of Wolbachia endosymbiont transferred to X chromosome of host insect". Proc. Natl. Acad. Sci. U.S.A. 99 (22): 14280–85. PMID 12386340.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sprague G (1991). "Genetic exchange between kingdoms". Curr. Opin. Genet. Dev. 1 (4): 530–33. PMID 1822285.
- ^ Baldo A, McClure M (1999). "Evolution and horizontal transfer of dUTPase-encoding genes in viruses and their hosts". J. Virol. 73 (9): 7710–21. PMID 10438861.
- ^ Poole A, Penny D (2007). "Evaluating hypotheses for the origin of eukaryotes". Bioessays. 29 (1): 74–84. PMID 17187354.
- ^ Lande R (1989). "Fisherian and Wrightian theories of speciation". Genome. 31 (1): 221–27. PMID 2687093.
- ^ Otto S, Whitlock M (1997). "The probability of fixation in populations of changing size". Genetics. 146 (2): 723–33. PMID 9178020.
- ^ Nei M (2005). "Selectionism and neutralism in molecular evolution". Mol. Biol. Evol. 22 (12): 2318–42. PMID 16120807.
- ^ Kimura M (1991). "The neutral theory of molecular evolution: a review of recent evidence". Jpn. J. Genet. 66 (4): 367–86. PMID 1954033.
- ^ Kimura M (1989). "The neutral theory of molecular evolution and the world view of the neutralists". Genome. 31 (1): 24–31. PMID 2687096.
- ^ Darwin, Charles (1859). On the Origin of Species (1st ed.). London: John Murray. pp. p. 1.
{{cite book}}:|pages=has extra text (help). Related earlier ideas were acknowledged in Darwin, Charles (1861). On the Origin of Species (3rd ed.). London: John Murray. pp. p. xiii.{{cite book}}:|pages=has extra text (help) - ^ Orr H (2005). "The genetic theory of adaptation: a brief history". Nat. Rev. Genet. 6 (2): 119–27. PMID 15716908.
- ^ Nakajima A, Sugimoto Y, Yoneyama H, Nakae T (2002). "High-level fluoroquinolone resistance in Pseudomonas aeruginosa due to interplay of the MexAB-OprM efflux pump and the DNA gyrase mutation". Microbiol. Immunol. 46 (6): 391–95. PMID 12153116.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Gould 2002, pp. 1235–1236
- ^ Bejder L, Hall BK (2002). "Limbs in whales and limblessness in other vertebrates: mechanisms of evolutionary and developmental transformation and loss". Evol. Dev. 4 (6): 445–58. PMID 12492145.
- ^ Salesa MJ, Antón M, Peigné S, Morales J (2006). "Evidence of a false thumb in a fossil carnivore clarifies the evolution of pandas". Proc. Natl. Acad. Sci. U.S.A. 103 (2): 379–82. PMID 16387860.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Fong D, Kane T, Culver D (1995). "Vestigialization and Loss of Nonfunctional Characters". Ann. Rev. Ecol. Syst. 26: 249–68. doi:10.1146/annurev.es.26.110195.001341.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jeffery WR (2005). "Adaptive evolution of eye degeneration in the Mexican blind cavefish". J. Hered. 96 (3): 185–96. PMID 15653557.
- ^ Maxwell EE, Larsson HC (2007). "Osteology and myology of the wing of the Emu (Dromaius novaehollandiae), and its bearing on the evolution of vestigial structures". J. Morphol. 268 (5): 423–41. PMID 17390336.
- ^ Bejder L, Hall BK (2002). "Limbs in whales and limblessness in other vertebrates: mechanisms of evolutionary and developmental transformation and loss". Evol. Dev. 4 (6): 445–58. PMID 12492145.
- ^ Silvestri AR, Singh I (2003). "The unresolved problem of the third molar: would people be better off without it?". Journal of the American Dental Association (1939). 134 (4): 450–55. PMID 12733778.
- ^ Johnson NA, Porter AH (2001). "Toward a new synthesis: population genetics and evolutionary developmental biology". Genetica. 112–113: 45–58. PMID 11838782.
- ^ Baguñà J, Garcia-Fernàndez J (2003). "Evo-Devo: the long and winding road". Int. J. Dev. Biol. 47 (7–8): 705–13. PMID 14756346.
*Gilbert SF (2003). "The morphogenesis of evolutionary developmental biology". Int. J. Dev. Biol. 47 (7–8): 467–77. PMID 14756322. - ^ Allin EF (1975). "Evolution of the mammalian middle ear". J. Morphol. 147 (4): 403–37. PMID 1202224.
- ^ Harris MP, Hasso SM, Ferguson MW, Fallon JF (2006). "The development of archosaurian first-generation teeth in a chicken mutant". Curr. Biol. 16 (4): 371–77. PMID 16488870.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Gavrilets S (2003). "Perspective: models of speciation: what have we learned in 40 years?". Evolution. 57 (10): 2197–215. PMID 14628909.
- ^ Jiggins CD, Bridle JR (2004). "Speciation in the apple maggot fly: a blend of vintages?". Trends Ecol. Evol. (Amst.). 19 (3): 111–4. PMID 16701238.
*Boxhorn, J (1995). "Observed Instances of Speciation". The TalkOrigins Archive. Retrieved 2007-05-10.
*Weinberg JR, Starczak VR, Jorg, D (1992). "Evidence for Rapid Speciation Following a Founder Event in the Laboratory". Evolution. 46 (4): 1214–20. doi:10.2307/2409766.{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hoskin CJ, Higgle M, McDonald KR, Moritz C (2005). "Reinforcement drives rapid allopatric speciation". Nature. 437: 1353–356. doi:10.1038/nature04004.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Templeton AR (1980). "The theory of speciation via the founder principle". Genetics. 94 (4): 1011–38. PMID 6777243.
- ^ Antonovics J (2006). "Evolution in closely adjacent plant populations X: long-term persistence of prereproductive isolation at a mine boundary". Heredity. 97 (1): 33–37. PMID 16639420.
- ^ Nosil P, Crespi B, Gries R, Gries G (2007). "Natural selection and divergence in mate preference during speciation". Genetica. 129 (3): 309–27. PMID 16900317.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Savolainen V, Anstett M-C, Lexer C, Hutton I, Clarkson JJ, Norup MV, Powell MP, Springate D, Salamin N, Baker WJr (2006). "Sympatric speciation in palms on an oceanic island". Nature. 441: 210–13. PMID 16467788.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
*Barluenga M, Stölting KN, Salzburger W, Muschick M, Meyer A (2006). "Sympatric speciation in Nicaraguan crater lake cichlid fish". Nature. 439: 719–723. PMID 16467837.{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gavrilets S (2006). "The Maynard Smith model of sympatric speciation". J. Theor. Biol. 239 (2): 172–82. PMID 16242727.
- ^ Belderok, Bob & Hans Mesdag & Dingena A. Donner. (2000) Bread-Making Quality of Wheat. Springer. p.3. ISBN 0-7923-6383-3.
*Hancock, James F. (2004) Planti Evolution and the Origin of Crop Species. CABI Publishing. ISBN 0-85199-685-X. - ^ Albertin W, Brabant P, Catrice O, Eber F, Jenczewski E, Chèvre AM, Thiellement H (2005). "Autopolyploidy in cabbage (Brassica oleracea L.) does not alter significantly the proteomes of green tissues". Proteomics. 5 (8): 2131–39. PMID 15852348.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ayala FJ, Escalante AA (1996). "The evolution of human populations: a molecular perspective". Mol. Phylogenet. Evol. 5 (1): 188–201. PMID 8673287.
- ^ Gould SJ (1994). "Tempo and mode in the macroevolutionary reconstruction of Darwinism". Proc. Natl. Acad. Sci. U.S.A. 91 (15): 6764–71. PMID 8041695.
- ^ a b c d Michael Pollan The Botany of Desire: A Plant's-eye View of the World. Bloomsbury. ISBN 0-7475-6300-4.
- ^ Matzke, Nicholas J. and Paul R. Gross. 2006. Analyzing Critical Analysis: The Fallback Antievolutionist Strategy. In Eugenie Scott and Glenn Branch, Not in Our Classrooms: Why Intelligent Design is Wrong for Our Schools, Beacon Press, Boston ISNB:0807032786
- ^ Dobzhansky, Theodosius Grigorievich (1937). Genetics and the origin of species. LC QH366 .D6.
{{cite book}}: Unknown parameter|Publisher=ignored (|publisher=suggested) (help), p12 - ^ a b c Erwin DH, Macroevolution is more than repeated rounds of microevolution, Department of Paleobiology, National Museum of Natural History, Washington, DC 20560, USA.