This article needs additional citations for verification. (December 2010) |
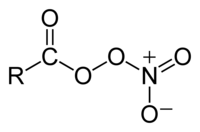
Peroxyacyl nitrates (also known as Acyl peroxy nitrates, APN or PANs) are powerful respiratory and eye irritants present in photochemical smog. They also facilitate the transport of air pollutants to areas away from the pollution sources.
Peroxyacetyl nitrate
edit| Names | |
|---|---|
| IUPAC name
nitroethaneperoxoate
| |
| Systematic IUPAC name
ethanoic nitric peroxyanhydride | |
| Other names
PAN
peroxyacetyl nitrate α-oxoethylperoxylnitrate | |
| Identifiers | |
3D model (JSmol)
|
|
| UNII | |
| |
| |
| Properties | |
| C2H3NO5 | |
| Molar mass | 121.05 g mol−1 |
| 1.46 × 10 5 mg l−1 at 298 K | |
| log P | −0.19 |
| Vapor pressure | 29.2 mmHg at 298 K |
Henry's law
constant (kH) |
0.000278 m³ atm mol−1 at 298 K |
Atmospheric OH rate constant
|
10−13 cm³ molecule−1 s−1 at 298 K |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Peroxyacetyl nitrate is is a secondary pollutant present in photochemical smog. It is thermally unstable and decomposes into peroxyethanoyl radicals and nitrogen dioxide gas. It is a lachrymatory substance.
Peroxyacetyl nitrate, or PAN, is an oxidant more stable than ozone. Hence, it is better capable of long-range transport than ozone. It serves as a carrier for oxides of nitrogen (NOx) into rural regions and causes ozone formation in the global troposphere.
The formation of PAN on a secondary scale becomes an issue when ethanol is used as an automotive fuel. Acetaldehyde emissions increase, which subsequently react in the atmosphere to form smog. Whereas ethanol policies solve domestic oil supply problems, they drastically exacerbate air quality conditions.[citation needed]
Formation and impact in the atmosphere
editPANs are produced in the thermal equilibrium between organic peroxy radicals by the gas-phase oxidation of a variety of volatile organic compounds (VOCs), or by aldehydes and other oxygenated VOCs oxidizing in the presence of NO2. For example, peroxyacetyl nitrate, CH3COOONO2:
Hydrocarbons + O2 + NO2 + light → CH3COOONO2
The general equation is;
CxHyO3 + NO2 → CxHyO3NO2
They are good markers for the source of VOCs as either biogenic or anthropogenic, which is useful in the study of global and local effects of pollutants.[1][2]
PANs are both toxic and irritating, as they dissolve more readily in water than ozone. They are lachrymators, causing eye irritation at concentrations of only a few parts per billion. At higher concentrations they cause extensive damage to vegetation. Both PANs and their chlorinated derivates are said to be mutagenic, as they can be a factor causing skin cancer.
PANs are secondary pollutants, which means they are not directly emitted as exhaust from power plants or internal combustion engines, but they are formed from other pollutants by chemical reactions in the atmosphere. Free radical reactions catalyzed by ultraviolet light from the sun oxidize unburned hydrocarbons to aldehydes, ketones, and dicarbonyl compounds, whose secondary reactions create peroxyacyl radicals, which combine with nitrogen dioxide to form peroxyacyl nitrates.
The most common peroxyacyl radical is peroxyacetyl, which can be formed from the free radical oxidation of acetaldehyde, various ketones, or the photolysis of dicarbonyl compounds such as methylglyoxal or diacetyl.
Since they dissociate quite slowly in the atmosphere into radicals and NO2, PANs are able to transport these unstable compounds far away from the urban and industrial origin. This is important for tropospheric ozone production as PANs transport NOx to regions where it can more efficiently produce ozone.
References
edit- ^ LaFranchi, B. W.; Wolfe, G. M. (2009). "Closing the peroxy acetyl nitrate budget: observations of acyl peroxy nitrates (PAN, PPN, and MPAN) during BEARPEX 200". Atmospheric Chemistry and Physics. 9 (19). Copernicus Publications: 7623–7641. doi:10.5194/acp-9-7623-2009.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Joel Thornton, Department of Atmospheric Sciences, University of Washington (14 November 2010). "PANs". Personal Website. N/A. Retrieved 14 November 2010.
{{cite web}}: CS1 maint: multiple names: authors list (link)