 | |
| Clinical data | |
|---|---|
| Trade names | Brukinsa |
| Other names | BGB-3111 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a620009 |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Bruton's tyrosine kinase (BTK) inhibitor[1] |
| Legal status | |
| Legal status |
|
| Chemical and physical data | |
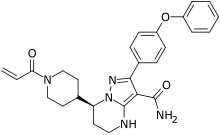
| Formula | C27H29N5O3 |
| Molar mass | 471.5509 g·mol−1 |
| 3D model (JSmol) | |
| |
Zanubrutinib, sold under the brand name Brukinsa, is a medication used for the treatment of mantle cell lymphoma (MCL) which has received prior treatment.[1] It may also be used for lymphoplasmatic lymphoma.[3] It is taken by mouth.[1]
Common side effects include low white blood cells, low platelets, rash, diarrhea, and low hemoglobin.[1] Other side effects may include bleeding, infection, atrial fibrillation, and another cancer.[1] Use during pregnancy may harm the baby.[1] It is a Bruton's tyrosine kinase (BTK) inhibitor and works by slowing tumor growth.[1]
Zanubrutinib was approved for medical use in the United States in 2019.[1] It received an orphan designation in Europe that year as well.[3] In the United States a month of treatment costs about 13,000 USD as of 2021.[4] This amount in China cost about 21,000 RMB (3,250 USD).[5]
References edit
- ^ a b c d e f g h i j k "Zanubrutinib Monograph for Professionals". Drugs.com. Archived from the original on 16 January 2021. Retrieved 4 August 2021.
- ^ "Zanubrutinib". DrugBank. Archived from the original on 15 November 2019. Retrieved 15 November 2019.
- ^ a b c "EU/3/19/2167". Archived from the original on 27 August 2021. Retrieved 4 August 2021.
- ^ "Zanubrutinib Prices and Zanubrutinib Coupons - GoodRx". GoodRx. Retrieved 4 August 2021.
- ^ "Application for the addition of Zanubrutinib on the WHO Model List of Essential Medicines" (PDF). November 2020. Archived (PDF) from the original on 27 August 2021. Retrieved 4 August 2021.