 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Temodar, Temodal, Temcad, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601250 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous |
| Drug class | Alkylating agent[2] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | almost 100% |
| Protein binding | 15% (10–20%) |
| Metabolism | hydrolysis |
| Metabolites | 3-methyl-(triazen-1-yl)imidazole-4-carboxamide (MTIC, the active species); temozolomide acid |
| Elimination half-life | 1.8 hours |
| Excretion | mainly kidney |
| Identifiers | |
| |
| Chemical and physical data | |
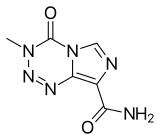
| Formula | C6H6N6O2 |
| Molar mass | 194.154 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 212 °C (414 °F) (decomp.) |
| |
| |
| | |
Temozolomide (TMZ), sold under the brand name Temodar among others, is a medication used to treat brain tumors such as glioblastoma and anaplastic astrocytoma.[2] It is taken by mouth or by gradual injection into a vein.[2]
Common side include nausea, vomiting, constipation, loss of appetite, hair loss, headache, tiredness, seizures, rash, low white blood cells, and low platelet.[2] People receiving the injection may also have pain, irritation, itching, redness, or bruising at the site.[2] Other side effects may include Pneumocystis jiroveci pneumonia, other cancers, and hepatitis B reactivation.[4] It is an alkylating agent.[2]
Temozolomide was approved for medical use in the United States and Europe in 1999.[4][2] It is available as a generic medication.[5] In the United Kingdom five pills of 250 mg costs the NHS about £530 as of 2021.[5] This amount in the United States costs about 135 USD.[6]
References edit
- ^ "Temozolomide". Drugs.com. 4 May 2020. Archived from the original on 29 August 2021. Retrieved 7 May 2020.
- ^ a b c d e f g h i "Temodal EPAR". European Medicines Agency (EMA). Archived from the original on 22 October 2020. Retrieved 7 May 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "Temodal Capsules - Summary of Product Characteristics (SmPC)". (emc). 24 October 2019. Archived from the original on 20 September 2020. Retrieved 7 May 2020.
- ^ a b "Temozolomide Monograph for Professionals". Drugs.com. Archived from the original on 29 August 2021. Retrieved 25 September 2021.
- ^ a b BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 949. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ^ "Temozolomide Prices and Temozolomide Coupons - GoodRx". GoodRx. Retrieved 25 September 2021.