 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Sutent, others |
| Other names | SU11248 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607052 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Receptor tyrosine kinase (RTK) inhibitor[1] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Unaffected by food |
| Protein binding | 95% |
| Metabolism | Liver (CYP3A4-mediated) |
| Elimination half-life | 40 to 60 hours (sunitinib) 80 to 110 hours (metabolite) |
| Excretion | Fecal (61%) and Kidney (16%) |
| Identifiers | |
| |
| Chemical and physical data | |
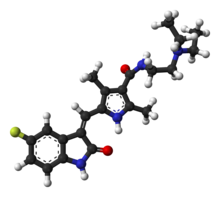
| Formula | C22H27FN4O2 |
| Molar mass | 398.482 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Sunitinib, sold under the brand name Sutent, is a medication used to treat cancer, specifically renal cell carcinoma (RCC), gastrointestinal stromal tumor (GIST), and pancreatic neuroendocrine tumor (pNET).[2] It is taken by mouth.[2]
Common side effects include tiredness, fever, nausea, diarrhea, inflammation of the mouth, abdominal pain, swelling, rash, pain, bleeding, and shortness of breath.[1] Other side effects may include liver problems, osteonecrosis of the jaw, and prolonged QT.[1] Use during pregnancy may harm the baby.[1] It is a receptor tyrosine kinase (RTK) inhibitor.[1]
Sunitinib was approved for medical use in the United States and Europe in 2006.[1][3] In the United Kingdom 4 weeks of medication costs the NHS about £3,100 as of 2021.[4] In the United States this amount costs about 4,900 USD.[5]
References edit
- ^ a b c d e f g "SUNItinib Monograph for Professionals". Drugs.com. Archived from the original on 23 January 2021. Retrieved 15 October 2021.
- ^ a b c d e "Sutent- sunitinib malate capsule". DailyMed. Archived from the original on 23 March 2021. Retrieved 7 April 2021.
- ^ "Sutent". Archived from the original on 12 July 2021. Retrieved 15 October 2021.
- ^ BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 1058. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ^ "Sunitinib Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 15 October 2021.