 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌdɒksɪˈsaɪkliːn/ DOKS-i-SY-kleen |
| Trade names | Doryx, Doxyhexal, Doxylin among others |
| AHFS/Drugs.com | Systemic: Monograph Mouth: Monograph |
| MedlinePlus | a682063 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, IV[1] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~100% |
| Protein binding | 80–90% |
| Metabolism | Negligible |
| Elimination half-life | 10–22 hours |
| Excretion | Mainly faeces, 40% urine |
| Identifiers | |
| |
| Chemical and physical data | |
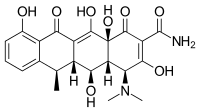
| Formula | C22H24N2O8 |
| Molar mass | 444.440 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Doxycycline is an antibiotic used in the treatment of infections caused by bacteria and certain parasites.[1] It is used to treat bacterial pneumonia, acne, chlamydia infections, early Lyme disease, cholera, typhus, and syphilis.[1] It is also used to prevent malaria and in combination with quinine, to treat malaria.[1] Doxycycline may be taken by mouth or by injection into a vein.[1]
Common side effects include diarrhea, nausea, vomiting, and an increased risk of sunburn.[1] There have been concerns in the first trimester of pregnancy or in young children may result in permanent discoloration of the teeth.[1] Though in children this does not occur with normal doses.[3] Its use during breastfeeding is probably safe.[1] Doxycycline is a broad-spectrum antibiotic, of the tetracycline class.[1] Like other agents of this class, it either slows or kills bacteria by inhibiting protein production.[1][4] It kills malaria by targeting a plastid organelle, the apicoplast.[5][6]
Doxycycline was patented in 1957 and came into commercial use in 1967.[7][8] It is on the World Health Organization's List of Essential Medicines.[9] Doxycycline is available as a generic medicine and is generally inexpensive.[1][10] The wholesale cost in the developing world is between US$0.01 and US$0.04 per pill.[11] In the United States, ten days of treatment has a wholesale costs of about US$3.40 as of 2019.[12] However, in 2014, due to supply issues, it was being sold for as much as US$60–200 for that amount.[1][13] In 2017, it was the 113th most commonly prescribed medication in the United States, with more than six million prescriptions.[14][15]
References edit
- ^ a b c d e f g h i j k l "Doxycycline calcium". The American Society of Health-System Pharmacists. Archived from the original on 23 September 2015. Retrieved 18 August 2015.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 2 July 2020. Retrieved 22 September 2020.
- ^ Ravindra, D; Huang, G; Hallett, K; Burgner, DP; Gwee, A; Silva, MJ (1 July 2023). "Antibiotic Exposure and Dental Health: A Systematic Review". Pediatrics. 152 (1). doi:10.1542/peds.2023-061350. PMID 37264510.
- ^ Nelson, ML; Levy, SB (December 2011). "The history of the tetracyclines". Annals of the New York Academy of Sciences. 1241 (1): 17–32. Bibcode:2011NYASA1241...17N. doi:10.1111/j.1749-6632.2011.06354.x. PMID 22191524.
- ^ McFadden GI (March 2014). "Apicoplast". Curr. Biol. 24 (7): R262–3. doi:10.1016/j.cub.2014.01.024. PMID 24698369.
- ^ Schlagenhauf-Lawlor, Patricia (2008). Travelers' Malaria. PMPH-USA. p. 148. ISBN 9781550093360. Archived from the original on 1 August 2020. Retrieved 18 August 2019.
- ^ Fischer, Janos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 489. ISBN 9783527607495. Archived from the original on 1 August 2020. Retrieved 9 September 2017.
- ^ Corey, E.J. (2013). Drug discovery practices, processes, and perspectives. Hoboken, N.J.: John Wiley & Sons. p. 406. ISBN 9781118354469. Archived from the original on 1 August 2020. Retrieved 9 September 2017.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Hamilton, Richard J. (2011). Tarascon pharmacopoeia (12th ed.). Sudbury, MA: Jones & Bartlett Learning. p. 79. ISBN 9781449600679. Archived from the original on 1 August 2020. Retrieved 9 September 2017.
- ^ "Doxycycline". International Medical Products Price Guide. Archived from the original on 30 March 2019. Retrieved 16 January 2018.
- ^ "NADAC as of 2019-09-11 | Data.Medicaid.gov". Centers for Medicare and Medicaid Services. Archived from the original on 30 December 2019. Retrieved 10 September 2019.
- ^ "Officials Question the Rising Costs of Generic Drugs". New York Times. 7 October 2014. Archived from the original on 28 October 2015. Retrieved 22 September 2015.
- ^ "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ^ "Doxycycline - Drug Usage Statistics". ClinCalc. Archived from the original on 8 July 2020. Retrieved 11 April 2020.