 | |
| Combination of | |
|---|---|
| Diphenhydramine | Antiemetic |
| 8-chlorotheophylline | Stimulant |
| Clinical data | |
| Pronunciation | dye" men hye' dri nate[1] |
| Trade names | Draminate, Gravol, Dramamine, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607046 |
| Pregnancy category |
|
| Routes of administration | By mouth, rectal, intravenous, intramuscular[3] |
| Drug class | First generation antihistamine[1] |
| Legal status | |
| Legal status | |
| | |
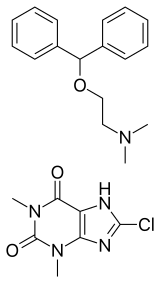
Dimenhydrinate, marketed as Dramamine and Gravol among others, is a medication used to treat motion sickness, nausea, and vertigo.[3] While it maybe useful for allergies it has not been studied for this condition.[3] It is a combination of diphenhydramine and 8-chlorotheophylline.[1][4]
Common side effects include sleepiness, blurry vision, dry mouth, headache, confusion, and dizziness.[3][1] Children may become hyperactive.[3] Other side effects may include urinary retention and glaucoma.[1] It has been taken by many pregnant women without any evidence of harm to the baby.[2] Low doses when breastfeeding appear safe.[2] Dimenhydrinate is a first generation antihistamine.[1] It works mostly via the effects of diphenhydramine which blocks acetylcholine.[3] The 8-chlorotheophylline is present to try to decrease the side effect of sleepiness.[1]
Dimenhydrinate has been used medically since at least 1947 after being developed by G. D. Searle & Company.[5][6] It is avaliable over-the-counter drug and as a generic medication.[3] In the United States 120 tablets of 50 mg strength can be purchased for less than 10 USD as of 2020.[7] It may be used recreationally for the high that large doses can cause.[8][9]
References edit
- ^ a b c d e f g h "Dimenhydrinate". LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. January 16, 2017. Archived from the original on 28 August 2021. Retrieved 10 September 2020.
- ^ a b c d "Dimenhydrinate Use During Pregnancy". Drugs.com. Retrieved 10 September 2020. Cite error: The named reference "Preg2020" was defined multiple times with different content (see the help page).
- ^ a b c d e f g h i "Dimenhydrinate Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 18 October 2020. Retrieved 10 September 2020.
- ^ Putra, Okky Dwichandra; Yoshida, Tomomi; Umeda, Daiki; Higashi, Kenjirou; Uekusa, Hidehiro; Yonemochi, Etsuo (29 July 2016). "Crystal Structure Determination of Dimenhydrinate after More than 60 Years: Solving Salt–Cocrystal Ambiguity via Solid-State Characterizations and Solubility Study". Crystal Growth & Design. 16 (9): 5223–5229. doi:10.1021/acs.cgd.6b00771.
- ^ Sneader, Walter. Drug Discovery: A History. John Wiley & Sons. p. 405. ISBN 978-0-471-89979-2. Archived from the original on 2021-08-28. Retrieved 2020-09-10.
- ^ Root, Walter S.; Hofmann, Frederick G. The Nervous System: Central Nervous System Drugs. Elsevier. p. 311. ISBN 978-1-4832-7583-3. Archived from the original on 2021-08-28. Retrieved 2020-09-10.
- ^ "Compare Dimenhydrinate Prices". GoodRx. Retrieved 10 September 2020.
- ^ Hoffman, Robert S.; Howland, Mary Ann; Lewin, Neal A.; Nelson, Lewis S.; Goldfrank, Lewis R. (2014). Goldfrank's Toxicologic Emergencies, Tenth Edition (ebook). McGraw Hill Professional. p. 667. ISBN 978-0-07-180185-0. Archived from the original on 2021-08-28. Retrieved 2020-10-31.
- ^ Gold, Jennifer. "ConsumerMedSafety.org - Prevent Medication Errors - Consumer Med Safety". consumermedsafety.org. Archived from the original on 21 January 2021. Retrieved 10 September 2020.