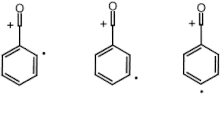
Distonic radical ions are unique due to the fact that its charges and radical sites are in different locations (on separate atoms ) ,unlike regular radicals where the formal charge and unpaired electron are in the same location. These molecular species are created when ionization takes place with either zwitterions or diradicals; ultimately, a neutral molecule loses an electron. [1] Through experimental research distonic radicals have been found to be extremely stable gas phase ions [2] and can be separated into different classes depending on the inherent features of the charged portion of the ion [3].
History
editIn 1984 scientists Bouma, Radom ,and Yates originated the term through extensive experimental research but they were not the first to deal with distonic ions. Experiments date back to the 1970s with Gross and McLafferty who were the first to propose the idea of such a species[4].
Ion Structure
editThere are several efficient techniques in place that can detect the presence of distonic ions, however when deciding on which method is most appropriate, one must keep in mind the ions internal energy and lifespan[2]. When a molecule is ionized and can structurally be classified as a distonic ion, the molecules kinetics and thermodynamic properties have been greatly altered. However, additional chemical properties are based on the reactions of the central excited ions. Mass spectrometry techniques are used to study their chemistry.[5]
Experimental Data
editDistonic ions have been extensively examined due to their unique behavior and how common they can occur.[1] Through the obtained data, it has been shown that in most cases distonic ions have corresponding bonding arrangement as the original molecule before ionization occurred. However data has also shown that distonic ions possess less stability than before ionization occurred; having said that, this does not take away from the fact that distonic ions are considered stable ions [2]
References
edit- 1) https://doi.org/10.1016/j.jasms.2006.03.008[1]
- 2) https://pubs.acs.org/doi/abs/10.1021/cr00015a008?journalCode=chreay[2]
- 3) https://doi.org/10.1016/S1044-0305(99)00053-7[3]
- 4) Properties and Reactivity of Gaseous Distonic Radical Ions with Aryl Radical Sites[4]
- 5) https://www.sciencedirect.com/science/article/pii/S0040402001880856?via%3Dihub
- ^ a b c Tomazela, Daniela Maria; Sabino, Adão A.; Sparrapan, Regina; Gozzo, Fabio C.; Eberlin, Marcos N. (2006-07-01). "Distonoid ions". Journal of the American Society for Mass Spectrometry. 17 (7): 1014–1022. doi:10.1016/j.jasms.2006.03.008. ISSN 1879-1123. PMID 16713292. S2CID 3667152.
- ^ a b c d Stirk, Krista M.; Kiminkinen, L. K. Marjatta; Kenttamaa, Hilkka I. (1992-11-01). "Ion-molecule reactions of distonic radical cations". Chemical Reviews. 92 (7): 1649–1665. doi:10.1021/cr00015a008. ISSN 0009-2665.
- ^ a b Hill, Brian T.; Poutsma, John C.; Chyall, Leonard J.; Hu, Jun; Squires, Robert R. (1999-09-01). "Distonic ions of the "ate" class". Journal of the American Society for Mass Spectrometry. 10 (9): 896–906. doi:10.1016/S1044-0305(99)00053-7. ISSN 1879-1123. S2CID 97267758.
- ^ a b Williams, Peggy E.; Jankiewicz, Bartlomiej J.; Yang, Linan; Kenttamaa, Hilkka I. (2013-10-24). "ChemInform Abstract: Properties and Reactivity of Gaseous Distonic Radical Ions with Aryl Radical Sites". ChemInform. 44 (46): no. doi:10.1002/chin.201346233. ISSN 0931-7597.
- ^ https://www.sciencedirect.com/science/article/pii/S0040402001880856.
{{cite web}}: Missing or empty|title=(help)
This is a user sandbox of Mailangrichardson. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |
This is a user sandbox of Mailangrichardson. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |