pH sensitive or pH responsive polymers are materials which will respond to the changes in the pH of the surrounding medium by varying their dimensions. Materials may swell, collapse, or change depending on the pH of their environment. This behavior is exhibited due to the presence of certain functional groups in the polymer chain. pH-sensitive materials can be either acidic or basic, responding to either basic or acidic pH values. These polymers can be designed with many different architectures for different applications. Key uses of pH sensitive polymers are controlled drug delivery systems, biomimetics, micromechanical systems, separation processes, and surface functionalization.
Types
pH sensitive polymers can be broken into two categories: those with acidic groups (such as -COOH and -SO3H) and those with basic groups (-NH2). The mechanism of response is the same for both, only the stimulus varies. The general form of the polymer is a backbone with functional "pendant groups" that hang off of it. When these functional groups become ionized in certain pH levels, they acquire a charge (+/-). Repulsions between like charges cause the polymers to change shape.[1][2]
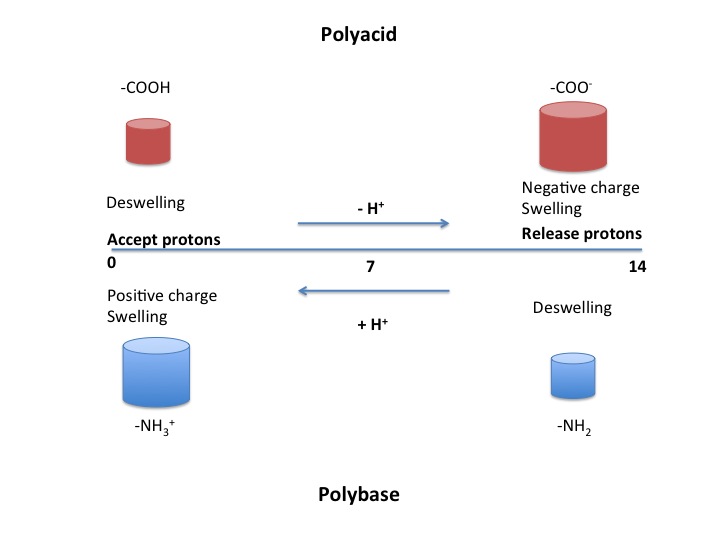
Polyacids
Polyacids, also known as anionic polymers, are polymers that have acidic groups.[2] Examples of acidic functional groups include carboxylic acids (-COOH), sulfonic acids (-SO3H), phosphonic acids, and boronic acids. Polyacids accept protons at low pH values. At higher pH values, they deprotonate and become negatively charged.[1] The negative charges create a repulsion that causes the polymer to swell. This swelling behavior is observed when the pH is greater than the pKa of the polymer.[2]

Polybases
Polybases are the basic equivalent of polyacids and are also known as cationic polymers. They accept protons at low pH like polyacids do, but they then become positively charged. In contrast, at higher pH values they are neutral. Swelling behavior is seen when the pH is less than the pKa of the polymer.[2]
Natural polymers
Although many sources talk about synthetic pH sensitive polymers, natural polymers can also display pH-responsive behavior. Examples include chitrosan, hyaluronic acid, and dextran.[1] Chitosan, a frequently used example, is cationic. Since DNA is negatively charged, DNA could be attached to chitosan as a way to deliver genes to cells.[3] Natural polymers have appeal because they display good biocompatibility, which makes them useful for biomedical applications. However, a disadvantage to natural polymers is that researchers can have more control over the structure of synthetic polymers and so can design those polymers for specific applications.[2]



Multi-stimuli polymers
Polymers can be designed to respond to more than one external stimulus, such as pH and temperature. Often, these polymers are structured as a copolymer where each polymer displays one type of response.[1]
Architectures
pH sensitive polymers have been created with linear block copolymer, star, branched, dendrimer, brush, and comb architectures. Polymers of different architectures will self-assemble into different structures. This self-assembly can occur due to the nature of the polymer and the solvent, or due to a change in pH. pH changes can also cause the larger structure to swell or deswell. For example, block copolymers often form micelles, as will star polymers and branched polymers. However, star and branched polymers can form rod or worm-shaped micelles rather than the typical spheres. Brush polymers are usually used for modifying surfaces since their structure doesn’t allow them to form a larger structure like a micelle.[1]
The architecture of a pH sensitive polymer is important because it can affect the practical applications of the polymer. For example, researchers made copolymers of poly(ε-caprolactone)-b-poly(N,N-diethylaminoethylmethacrylate)-r-poly(N-(3-sulfopropyl)-N-methacryloxyethy-N,N-diethylammoniumbetaine) (shortened to PCL-PDEASB) with linear and four-armed star architectures. The copolymers had the same composition, but the researchers observed different water contact angles (used to indicate hydrophobicity of a surface) and different drug release patterns for the different architectures.[4]
Response to change in pH
Often, the response to different pH values is a swelling or deswelling response. For example, polyacids release protons to become negatively charged at high pH. Since polymer chains are often in close proximity to other parts of the same chain or to other chains, like-charged parts of the polymer repel each other. This repulsion leads to a swelling of the polymer.[3]
Polymers can also form micelles (spheres) in response to a change in pH. This behavior can occur with linear block copolymers. If the different blocks of the copolymer have different properties, they can form micelles with one type of block on the inside and one type on the outside. For example, in water the hydrophobic blocks of a copolymer could end up on the inside of a micelle, with hydrophilic blocks on the outside. Additionally, a change in pH could cause micelles to swap their inner and outer molecules depending on the properties of the polymers involved.[1]
Responses other than simply swelling and deswelling with a change in pH are possible as well. Researchers created a copolymer that underwent a sol-gel transition (from a solution to a gel) in which the polymer was a gel from 2 < pH < 8. However, in that middle pH zone the researchers also observed that the gel softened at pH = 4.5 and then stiffened again at pH = 6.5. Subtle changes like this can be possible with a change in pH.[5]

Synthesis
Several different polymerization methods are used to make pH sensitive polymers. Living polymerization is often used because molecular weight distribution of the final polymers can be controlled. Examples include group transfer polymerization (GTP), atom transfer radical polymerization (ATRP), and reversible addition-fragmentation chain transfer (RAFT).[1] Graft copolymers are a popular type to synthesize because their structure is a backbone with branches. The composition of the branches can be changed to achieve different properties.[2]
Characterization of polymer properties
Contact angle measurements: Several methods can be used to measure the contact angle of a water drop on the surface of a polymer. The contact angle value is used to quantify wettability or hydrophobicity of the polymer.[2][6]
Degree of swelling: Equal to (swollen weight-deswelled weight)/deswelled weight *100% and determined by massing polymers before and after swelling. This indicates how much the polymer swelled upon a change in pH.[2]
pH critical point: the pH at which a significant structural change in how the molecules are arranged is observed. This structural change does not involve breaking bonds, but rather a change in conformation. For example, a swelling/deswelling transition would constitute a reversible conformational change. The value of the pH critical point can be determined by examining swelling percentage as a function of pH. Researchers aim to design molecules that transition at a pH that matters for the given application.[2][7]
Surface changes: confocal microscopy, scanning electron microscopy, Raman spectroscopy, and atomic force microscopy are all used to determine how the surface of a polymer changes in response to pH.[2]
Applications
Drug delivery
pH sensitive polymers have been researched extensively for use in drug delivery because different parts of the body display different pH values. For example, blood sits at a pH of 7.4-7.5, while the pH of the stomach is 1.0-3.0.[3] The goal is to design polymers with pH sensitivity that allows them to release a drug at the correct site. For example, since polyacids are neutral at lower pH and negatively charged at higher pH, they wouldn't swell in the acidic stomach but could swell and release a drug when they arrive at the less-acidic intestines.[7]
Hydrogels are a popular type of polymer considered for drug delivery. They can be produced in different shapes for different routes of delivery into the body. Typically, the goal is to have the drug leach out when the hydrogel swells, but drug release could also occur upon contraction.[7]
Finally, although it is common to consider the swelling/deswelling behavior as the method of drug delivery, drugs could also be released by breaking bonds. If the drug in question was incorporated into the polymer with bonds that only broke under certain pH conditions, the drugs could break off at a desired location in the body.[1]
Gene delivery
Gene delivery relies on getting genes into the nucleus of a cell. Typically, this process occurs via endocytosis. However, endosomes have a pH of 5.5-6.5 that could damage the genes before they reach the nucleus. pH sensitive polymers could be used to break through the membrane of the endosome, so that genes make it into the cell before they are broken down.[7]
Micromechanical applications
pH sensitive polymers have been utilized in several micromechanical systems. For example, researchers designed microvalves using a pH sensitive hydrogel consisting of acrylic acid and 2-hydroxyethyl methacrylate. The hydrogel acted as the actuator of the valve. When solutions of different pH values flowed through channels around the valve, the hydrogel would swell and block off the channels depending on the pH.[8] Other researchers used a hydrogel to deflect a cantilever. They made a silicon cantilever with a polymer consisting of poly(methacrylic acid) and poly(ethylene glycol) dimethacrylate on top. The researchers measured the swelling ratio of the polymers, measured as the (volume swollen/volume dry). The polymer swelled under a change in pH in the surrounding solution and caused the cantilever to bend. The deflection of the cantilever could be correlated with the pH of the solution.[9]
Purification and separation technology
pH sensitive polymers have been considered for use in membranes. A change in pH could change the ability of the polymer to let ions through, allowing it to act as a filter.[1]
Surface modification
pH sensitive polymers have been used to modify the surfaces of materials. For example, they can be used to change the wettability of a surface.[1] Researchers created a thin film of poly(N,N’-dimethylaminoethyl methacylate), also known as PDMAEMA. This polybase is positively charged and swells due to electrostatic repulsions at low pH. At high pH, it loses protons and becomes hydrophobic. The researchers used the contact angle of water droplets on the surface to quantify the wettability.[6]

- ^ a b c d e f g h i j Kocak, G.; Tuncer, C.; Bütün, V. (2016-12-20). "pH-Responsive polymers". Polymer Chemistry. 8 (1). doi:10.1039/C6PY01872F. ISSN 1759-9962.
- ^ a b c d e f g h i j Meléndez-Ortiz, H Iván; H.C. Varca (2016). "State of the art of smart polymers: from fundamentals to final applications". Polymer science: research advances, practical applications and educational aspects. Formatex Research Center. pp. 476–487.
- ^ a b c Almeida, Hugo; Helena Amaral, Maria; Lobão, Paulo (6/13/2012). "Temperature and pH stimuli-responsive polymers and their applications in controlled and self-regulated drug delivery" (PDF). Journal of Applied Pharmaceutical Science: 1–10.
{{cite journal}}: Check date values in:|date=(help) - ^ Wu, Zhengzhong; Cai, Mengtan; Xie, Xiaoxiong; He, Liu; Huang, Lei; Chen, Yuanwei; Luo, Xianglin (2015-12-14). "The effect of architecture/composition on the pH sensitive micelle properties and in vivo study of curcuminin-loaded micelles containing sulfobetaines". RSC Adv. 5 (129): 106989–107000. doi:10.1039/c5ra20847e. ISSN 2046-2069.
- ^ Popescu, Maria-Teodora; Tsitsilianis, Constantinos; Papadakis, Christine M.; Adelsberger, Joseph; Balog, Sandor; Busch, Peter; Hadjiantoniou, Natalie A.; Patrickios, Costas S. (2012-04-24). "Stimuli-Responsive Amphiphilic Polyelectrolyte Heptablock Copolymer Physical Hydrogels: An Unusual pH-Response". Macromolecules. 45 (8): 3523–3530. doi:10.1021/ma300222d. ISSN 0024-9297.
- ^ a b Zhang, Qiaolan; Xia, Fan; Sun, Taolei; Song, Wenlong; Zhao, Tianyi; Liu, Mancang; Jiang, Lei (2008-02-29). "Wettability switching between high hydrophilicity at low pH and high hydrophobicity at high pH on surface based on pH-responsive polymer". Chemical Communications (10). doi:10.1039/B716681H. ISSN 1364-548X.
- ^ a b c d Gil, Eun Seok; Hudson, Samuel M. (2004-12-01). "Stimuli-reponsive polymers and their bioconjugates". Progress in Polymer Science. 29 (12): 1173–1222. doi:10.1016/j.progpolymsci.2004.08.003.
- ^ Liu, R. H.; Yu, Qing; Beebe, D. J. (2002-02-01). "Fabrication and characterization of hydrogel-based microvalves". Journal of Microelectromechanical Systems. 11 (1): 45–53. doi:10.1109/84.982862. ISSN 1057-7157.
- ^ "Micromechanical cantilever as an ultrasensitive pH microsensor". Applied Physics Letters. 81 (16): 3091–3093. 2002-10-07. doi:10.1063/1.1514825. ISSN 0003-6951.