A cancer biomarker refers to a substance or process that is indicative of the presence of cancer in the body. A biomarker may be a molecule secreted by a tumor or a specific response of the body to the presence of cancer. Genetic, epigenetic, proteomic, glycomic, and imaging biomarkers can be used for cancer diagnosis, prognosis, and epidemiology. Ideally, such biomarkers can be assayed in non-invasively collected biofluids like blood or serum.[1]
While numerous challenges exist in translating biomarker research into the clinical space; a number of gene and protein based biomarkers have already been approved for use in patient care; including, AFP (Liver Cancer), BCR-ABL (Chronic Myeloid Leukemia), BRCA1 / BRCA2 (Breast/Ovarian Cancer), BRAF V600E (Melanoma/Colorectal Cancer), CA-125 (Ovarian Cancer) , CA19.9 (Pancreatic Cancer), CEA (Colorectal Cancer), EGFR (Non-small Cell Lung Cancer), HER-2 (Breast Cancer), KIT (Gastrointestinal Stromal Tumor), PSA (Prostate Specific Antigen), S100 (Melanoma), and many others.[2][3][4][5][6][7][8][9][10][11]
Definitions of Cancer Biomarkers edit
Different organizations and publications vary in their definition of biomarker. In many areas of medicine, biomarkers are limited to proteins identifiable or measurable in the blood or urine. However, the term is often used colloquially to cover any molecular, biochemical, physiological, or anatomical property that can be quantified and measured.
The National Cancer Institute (NCI), in particular, defines biomarker as a: “A biological molecule found in blood, other body fluids, or tissues that is a sign of a normal or abnormal process, or of a condition or disease. A biomarker may be used to see how well the body responds to a treatment for a disease or condition. Also called molecular marker and signature molecule."[12]
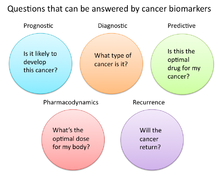
In cancer research and medicine, biomarkers are used in three primary ways:[13]
- To help diagnose conditions, as in the case of identifying early stage cancers (Diagnostic)
- To forecast how aggressive a condition is, as in the case of determining a patient's ability to fare in the absence of treatment (Prognostic)
- To predict how well a patient will respond to treatment (Predictive)
Role of Biomarkers in Cancer Research and Medicine edit
Uses of Biomarkers in Cancer Medicine edit
Risk Assessment edit
Cancer biomarkers, particular those associated with genetic mutations or epigenetic alterations, often offer a quantitative way to determine when individuals are predisposed to particular types of cancers. Notable examples of potentially predictive cancer biomarkers include mutations on genes KRAS, p53, EGFR, erbB2 for colorectal, esophageal, liver, and pancreatic cancer; mutations of genes BRCA1 and BRCA2 for breast and ovarian cancer; abnormal methylation of tumor suppressor genes p16, CDKN2B, and p14ARF for brain cancer; hypermethylation of MYOD1, CDH1, and CDH13 for cervical cancer; and hypermethylation of p16, p14, and RB1, for oral cancer.[14]
Diagnostic edit
Cancer biomarkers can also be useful in establishing a specific diagnosis. This is particularly the case when there is a need to determine whether tumors are of primary or metastatic origin. To make this distinction, researchers will screen the chromosomal alterations found on cells located in the primary tumor site against those found in the secondary site. If the alterations match, the secondary tumor can be identified as metastatic; whereas if the alterations differ, the secondary tumor can be identified as a distinct primary tumor.[15]
Prognostic edit
- These cancer biomarkers help to assess the risk of developing a particular cancer and determine prognosis. Because certain cancers progress more rapidly than others, these biomarkers help the doctors to decide how aggressive the cancer treatment should be. An example of this type of cancer biomarker is tissue inhibitor of metalloprotease-1 (TIMP1), and gives a better prognosis to the myeloma patients with lower levels of this protein.[16][17]
Predictive edit
- Predict the response to cancer drug(s) or treatment(s) in the patient.[18] An example of this is human epidermal growth factor receptor 2 (HER-2) overexpression due to an aberrant increase in the HER-2 gene. Breast cancer patients with this abnormal increase of HER-2 respond to trastuzumab (Herceptin®), a monoclonal antibody cancer drug. However, breast cancer patients not exhibiting the additional copies of the HER-2 gene are not recommended to go on this drug.[17]
Pharmacodynamics and Pharmacokinetics edit
- Cancer biomarkers under this category help determine the most effective dosage of drug or therapy is needed for that specific person.[19] These biomarkers are just another tool aiding the field of personalized medicine. An example is the thiopurine methyl-transferase (TPMT) gene.[17] Patients with mutations in the gene encoding TPMT are unable to metabolize large amounts of a leukemia drug, mercaptopurine, and this results in a possibly fatal drop in white blood cell count.[20] Because of the TPMT biomarker, cancer patients with a mutation can take a lower, and safer, dose of the cancer drug.
Recurrence edit
- Recurrence biomarkers are used to predict if cancer is likely to come back after treatment. An example is the Oncotype DX® breast cancer assay.[17][21] This assay looks at several genes within a breast tumor sample and quantitatively indicates the probability that the patient’s cancer will return.[22]
Roles for cancer biomarkers edit
Prognostic edit
- These cancer biomarkers help to assess the risk of developing a particular cancer and determine prognosis. Because certain cancers progress more rapidly than others, these biomarkers help the doctors to decide how aggressive the cancer treatment should be. An example of this type of cancer biomarker is tissue inhibitor of metalloprotease-1 (TIMP1), and gives a better prognosis to the myeloma patients with lower levels of this protein.[16][17]
Diagnostic edit
- Diagnose the particular type of cancer when pathologists are unable to identify the specific type of cancer from just looking at the cells.
Predictive edit
- Predict the response to cancer drug(s) or treatment(s) in the patient.[18] An example of this is human epidermal growth factor receptor 2 (HER-2) overexpression due to an aberrant increase in the HER-2 gene. Breast cancer patients with this abnormal increase of HER-2 respond to trastuzumab (Herceptin®), a monoclonal antibody cancer drug. However, breast cancer patients not exhibiting the additional copies of the HER-2 gene are not recommended to go on this drug.[17]
Pharmacodynamics and Pharmacokinetics edit
- Cancer biomarkers under this category help determine the most effective dosage of drug or therapy is needed for that specific person.[19] These biomarkers are just another tool aiding the field of personalized medicine. An example is the thiopurine methyl-transferase (TPMT) gene.[17] Patients with mutations in the gene encoding TPMT are unable to metabolize large amounts of a leukemia drug, mercaptopurine, and this results in a possibly fatal drop in white blood cell count.[20] Because of the TPMT biomarker, cancer patients with a mutation can take a lower, and safer, dose of the cancer drug.
Recurrence edit
- Recurrence biomarkers are used to predict if cancer is likely to come back after treatment. An example is the Oncotype DX® breast cancer assay.[17][21] This assay looks at several genes within a breast tumor sample and quantitatively indicates the probability that the patient’s cancer will return.[22]
Sensitivity and validity issues edit
Nothing is ever perfect, and cancer biomarkers also abide by this guideline. The sensitivity for a cancer biomarker is often debated because its reliability varies with the sensitivity of the biomarker. For example, if detection of lung cancer biomarker X could signify that ALL people with detectable levels of X would get lung cancer, but people without X would not develop lung cancer, then biomarker X would be the optimal lung cancer predictor. But in reality, there are going to be some false positives (which tell healthy people they will get lung cancer) and false negatives (which tell at-risk people they will not develop cancer). However, the markers with high sensitivity and accuracy would be key in early cancer prevention or detection. Moreover, the optimal cancer biomarker is one that can be easily accessed from the body (i.e. blood, urine, tissue from biopsy).
Types of cancer biomarkers edit
Molecular cancer biomarkers edit
| Tumor Type | Biomarker |
| Breast | ER (estrogen receptor)[23][24] |
| HER-2/neu [23][24] | |
| colorectal | EGFR [23][24] |
| KRAS [23][25] | |
| UGT1A1 [23][25] | |
| Gastric | HER-2/neu [23] |
| GIST | c-KIT [23][26] |
| Leukemia/Lymphoma | CD20 Antigen [23][27] |
| CD30 [23][28] | |
| FIP1L1-PDGRFalpha [23][29] | |
| PDGFR [23][30] | |
| Philadelphia Chromosome (BCR/ABL) [23][31][32] | |
| PML/RAR alpha [23][33] | |
| TPMT [23][34] | |
| UGT1A1 [23][35] | |
| Lung | ALK [23][36][37] |
| EGFR [23][24] | |
| KRAS [23][24] | |
| Melanoma | BRAF [23][37] |
Other Examples of Biomarkers:
- Tumor Suppressors Lost in Cancer
- RNA
- Proteins found in body fluids or tissue.
- Examples: Prostate-specific antigen, and CA-125
Imaging techniques edit
- Examples: MRI, Mammography [39]
- Imaging disease biomarkers by magnetic resonance imaging (MRI)
MRI has the advantages of having very high spatial resolution and is very adept at morphological imaging and functional imaging. MRI does have several disadvantages though. First, MRI has a sensitivity of around 10−3 mol/L to 10−5 mol/L which, compared to other types of imaging, can be very limiting. This problem stems from the fact that the difference between atoms in the high energy state and the low energy state is very small. For example, at 1.5 tesla, a typical field strength for clinical MRI, the difference between high and low energy states is approximately 9 molecules per 2 million. Improvements to increase MR sensitivity include increasing magnetic field strength, and hyperpolarization via optical pumping or dynamic nuclear polarization. There are also a variety of signal amplification schemes based on chemical exchange that increase sensitivity.
To achieve molecular imaging of disease biomarkers using MRI, targeted MRI contrast agents with high specificity and high relaxivity (sensitivity) are required. To date, many studies have been devoted to developing targeted-MRI contrast agents to achieve molecular imaging by MRI. Commonly, peptides, antibodies, or small ligands, and small protein domains, such as HER-2 affibodies, have been applied to achieve targeting. To enhance the sensitivity of the contrast agents, these targeting moieties are usually linked to high payload MRI contrast agents or MRI contrast agents with high relaxivities.[40]
References edit
- ^ Mishra, Alok (2010). "Cancer Biomarkers: Are We Ready for the Prime Time?". Cancers. 2 (1): 190–208. doi:10.3390/cancers2010190. PMID 24281040.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Rhea, Jeanne (March 2011). "Cancer Biomarkers: Surviving the journey from bench to bedside". Medical Laboratory Observer. Retrieved 26 April 2013.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: date and year (link) - ^ Behne, Tara; Copur, M. Sitki (1 January 2012). "Biomarkers for Hepatocellular Carcinoma". International Journal of Hepatology. 2012: 859076. doi:10.1155/2012/859076. PMC 3357951. PMID 22655201.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Musolino, A.; Bella, M. A.; Bortesi, B.; Michiara, M.; Naldi, N.; Zanelli, P.; Capelletti, M.; Pezzuolo, D.; Camisa, R.; Savi, M.; Neri, T. M.; Ardizzoni, A. (2007 Jun). "BRCA mutations, molecular markers, and clinical variables in early-onset breast cancer: a population-based study". Breast (Edinburgh, Scotland). 16 (3): 280–92. doi:10.1016/j.breast.2006.12.003. PMID 17257844.
{{cite journal}}: Check date values in:|date=(help) - ^ Dienstmann, R.; Tabernero, J. (2011 Mar). "BRAF as a target for cancer therapy". Anti-Cancer Agents in Medicinal Chemistry. 11 (3): 285–95. doi:10.2174/187152011795347469. PMID 21426297.
{{cite journal}}: Check date values in:|date=(help) - ^ Lamparella, N.; Barochia, A.; Almokadem, S. (2013). "Impact of genetic markers on treatment of non-small cell lung cancer". Advances in Experimental Medicine and Biology. 779: 145–64. doi:10.1007/978-1-4614-6176-0_6. ISBN 978-1-4614-6175-3. PMID 23288638.
- ^ Orphanos, G.; Kountourakis, P. (2012). "Targeting the HER2 receptor in metastatic breast cancer". Hematology/Oncology and Stem Cell Therapy. 5 (3): 127–37. doi:10.5144/1658-3876.2012.127. PMID 23095788.
- ^ Deprimo, Samuel E.; Huang, Xin; Blackstein, Martin E.; Garrett, Christopher R.; Harmon, Charles S.; Schöffski, Patrick; Shah, Manisha H.; Verweij, Jaap; Baum, Charles M.; Demetri, George D. (8 September 2009). "Circulating Levels of Soluble KIT Serve as a Biomarker for Clinical Outcome in Gastrointestinal Stromal Tumor Patients Receiving Sunitinib following Imatinib Failure". Clinical Cancer Research. 15 (18): 5869–5877. doi:10.1158/1078-0432.CCR-08-2480. PMID 19737953.
- ^ Bantis, A.; Grammaticos, P. (2012 Sep-Dec). "Prostatic specific antigen and bone scan in the diagnosis and follow-up of prostate cancer. Can diagnostic significance of PSA be increased?". Hellenic Journal of Nuclear Medicine. 15 (3): 241–6. PMID 23227460.
{{cite journal}}: Check date values in:|date=(help) - ^ Kruijff, S.; Hoekstra, H. J. (2012 Apr). "The current status of S-100B as a biomarker in melanoma". European Journal of Surgical Oncology : The Journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 38 (4): 281–5. doi:10.1016/j.ejso.2011.12.005. PMID 22240030.
{{cite journal}}: Check date values in:|date=(help) - ^ Ludwig, J. A.; Weinstein, J. N. (2005 Nov). "Biomarkers in cancer staging, prognosis and treatment selection". Nature Reviews. Cancer. 5 (11): 845–56. doi:10.1038/nrc1739. PMID 16239904.
{{cite journal}}: Check date values in:|date=(help) - ^ "biomarker". NCI Dictionary of Cancer Terms. National Cancer Institute.
- ^ "Biomarkers in Cancer: An Introductory Guide for Advocates" (PDF). Research Advocay Network. 2010. Retrieved 26 April 2013.
- ^ Verma, M.; Manne, U. (2006 Oct). "Genetic and epigenetic biomarkers in cancer diagnosis and identifying high risk populations". Critical Reviews in Oncology/Hematology. 60 (1): 9–18. doi:10.1016/j.critrevonc.2006.04.002. PMID 16829121.
{{cite journal}}: Check date values in:|date=(help) - ^ Leong, P. P.; Rezai, B.; Koch, W. M.; Reed, A.; Eisele, D.; Lee, D. J.; Sidransky, D.; Jen, J.; Westra, W. H. (1998 Jul 1). "Distinguishing second primary tumors from lung metastases in patients with head and neck squamous cell carcinoma". Journal of the National Cancer Institute. 90 (13): 972–7. doi:10.1093/jnci/90.13.972. PMID 9665144.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Terpos E, Dimopoulos MA, Shrivastava V; et al. (March 2010). "High levels of serum TIMP-1 correlate with advanced disease and predict for poor survival in patients with multiple myeloma treated with novel agents". Leuk. Res. 34 (3): 399–402. doi:10.1016/j.leukres.2009.08.035. PMID 19781774.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h http://www.researchadvocacy.org
- ^ a b Ludwig JA, Weinstein JN (November 2005). "Biomarkers in cancer staging, prognosis and treatment selection". Nat. Rev. Cancer. 5 (11): 845–56. doi:10.1038/nrc1739. PMID 16239904.
{{cite journal}}: CS1 maint: date and year (link) - ^ a b Sawyers CL (April 2008). "The cancer biomarker problem". Nature. 452 (7187): 548–52. doi:10.1038/nature06913. PMID 18385728.
{{cite journal}}: CS1 maint: date and year (link) - ^ a b Relling MV, Hancock ML, Rivera GK; et al. (December 1999). "Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus". J. Natl. Cancer Inst. 91 (23): 2001–8. doi:10.1093/jnci/91.23.2001. PMID 10580024.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ a b http://www.oncotypedx.com/HealthcareProfessional/Overview.aspx.
- ^ a b http://www.oncotypedx.com/en-US/Breast/PatientCaregiver/OncoOverview.aspx
- ^ a b c d e f g h i j k l m n o p q r s "Table of Pharmacogenomic Biomarkers in Drug Labels". U.S Food and Drug Administration.
- ^ a b c d e "Tumor Markers Fact Sheet" (PDF). American Cancer Society.
- ^ a b Lenz, Heinz-Josef. EdBk.GI.Colo.04.pdf "Established Biomarkers in Colon Cancer" (PDF). American Society of Clinical Oncology’s 2009 Educational Book.
{{cite web}}: Check|url=value (help) - ^ Gonzalez RS, Carlson G, Page AJ, Cohen C (July 2011). "Gastrointestinal stromal tumor markers in cutaneous melanomas: relationship to prognostic factors and outcome". Am. J. Clin. Pathol. 136 (1): 74–80. doi:10.1309/AJCP9KHD7DCHWLMO. PMID 21685034.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Tam CS, Otero-Palacios J, Abruzzo LV; et al. (April 2008). "Chronic lymphocytic leukaemia CD20 expression is dependent on the genetic subtype: a study of quantitative flow cytometry and fluorescent in-situ hybridization in 510 patients". Br. J. Haematol. 141 (1): 36–40. doi:10.1111/j.1365-2141.2008.07012.x. PMID 18324964.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Zhang M, Yao Z, Patel H; et al. (May 2007). "Effective therapy of murine models of human leukemia and lymphoma with radiolabeled anti-CD30 antibody, HeFi-1". Proc. Natl. Acad. Sci. U.S.A. 104 (20): 8444–8. doi:10.1073/pnas.0702496104. PMC 1895969. PMID 17488826.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Yamada Y, Sanchez-Aguilera A, Brandt EB; et al. (September 2008). "FIP1L1/PDGFRalpha synergizes with SCF to induce systemic mastocytosis in a murine model of chronic eosinophilic leukemia/hypereosinophilic syndrome". Blood. 112 (6): 2500–7. doi:10.1182/blood-2007-11-126268. PMID 18539901.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Nimer SD (May 2008). "Myelodysplastic syndromes". Blood. 111 (10): 4841–51. doi:10.1182/blood-2007-08-078139. PMID 18467609.
{{cite journal}}: CS1 maint: date and year (link) - ^ Ottmann O, Dombret H, Martinelli G; et al. (October 2007). "Dasatinib induces rapid hematologic and cytogenetic responses in adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia with resistance or intolerance to imatinib: interim results of a phase 2 study". Blood. 110 (7): 2309–15. doi:10.1182/blood-2007-02-073528. PMID 17496201.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Boulos N, Mulder HL, Calabrese CR; et al. (March 2011). "Chemotherapeutic agents circumvent emergence of dasatinib-resistant BCR-ABL kinase mutations in a precise mouse model of Philadelphia chromosome-positive acute lymphoblastic leukemia". Blood. 117 (13): 3585–95. doi:10.1182/blood-2010-08-301267. PMC 3072880. PMID 21263154.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ O'Connell PA, Madureira PA, Berman JN, Liwski RS, Waisman DM (April 2011). "Regulation of S100A10 by the PML-RAR-α oncoprotein". Blood. 117 (15): 4095–105. doi:10.1182/blood-2010-07-298851. PMID 21310922.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Duffy MJ, Crown J (November 2008). "A personalized approach to cancer treatment: how biomarkers can help". Clin. Chem. 54 (11): 1770–9. doi:10.1373/clinchem.2008.110056. PMID 18801934.
{{cite journal}}: CS1 maint: date and year (link) - ^ Ribrag V, Koscielny S, Casasnovas O; et al. (April 2009). "Pharmacogenetic study in Hodgkin lymphomas reveals the impact of UGT1A1 polymorphisms on patient prognosis". Blood. 113 (14): 3307–13. doi:10.1182/blood-2008-03-148874. PMID 18768784.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Li Y, Ye X, Liu J, Zha J, Pei L (January 2011). "Evaluation of EML4-ALK fusion proteins in non-small cell lung cancer using small molecule inhibitors". Neoplasia. 13 (1): 1–11. doi:10.1593/neo.101120. PMC 3022423. PMID 21245935.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ a b Pao W, Girard N (February 2011). "New driver mutations in non-small-cell lung cancer". Lancet Oncol. 12 (2): 175–80. doi:10.1016/S1470-2045(10)70087-5. PMID 21277552.
{{cite journal}}: CS1 maint: date and year (link) - ^ Bartels CL, Tsongalis GJ (April 2009). "MicroRNAs: novel biomarkers for human cancer". Clin. Chem. 55 (4): 623–31. doi:10.1373/clinchem.2008.112805. PMID 19246618.
{{cite journal}}: CS1 maint: date and year (link) - ^ Dalton WS, Friend SH (May 2006). "Cancer biomarkers—an invitation to the table". Science. 312 (5777): 1165–8. doi:10.1126/science.1125948. PMID 16728629.
{{cite journal}}: CS1 maint: date and year (link) - ^ Xue, Shenghui; Qiao, Jingjuan; Pu, Fan; Cameron, Mathew; Yang, Jenny J. (17). "Design of a novel class of protein-based magnetic resonance imaging contrast agents for the molecular imaging of cancer biomarkers". Wiley Interdiscip Rev Nanomed Nanobiotechnol. 5 (2): 163–179. doi:10.1002/wnan.1205. PMC 4011496. PMID 23335551.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|month=ignored (help)
External links edit
- CancerDriver: open database of cancer biomarkers with links to references and recruiting clinical trials.
- The latest news about oncology biomarkers