Tilisolol (INN, trade name Selecal) is a beta blocker.[1]
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
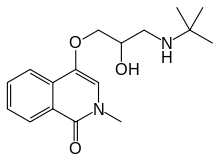
| Formula | C17H24N2O3 |
| Molar mass | 304.390 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Synthesis
editThe methanolysis of Phthalic anhydride [85-44-9] (1) gives Methyl hydrogen phthalate [4376-18-5] (2). Schotten-Baumann amidation with Methyl sarcosinate [5473-12-1] (3) gives Methyl 2-[(2-methoxy-2-oxoethyl)-methylcarbamoyl]benzoate, PC11644670 (4). Intramolecular lactamization with sodium methoxide afforded Methyl 4-hydroxy-2-methyl-1-oxoisoquinoline-3-carboxylate, PC54684295 (5). In lye saponification followed by decarboxylation occurred to give 4-hydroxy-2-methylisoquinolin-1(2H)-one [30236-50-1] (6). Treatment with Epichlorhydrin [106-89-8] (7) in the presence of base led to 2-Methyl-4-[(oxiran-2-yl)methoxy]isoquinolin-1(2H)-one [62775-08-0] (8). Opening of the oxirane ring with tert-Butylamine [75-64-9] (9) completed the synthesis of Tilisolol (10).
References
edit- ^ Imaizumi T, Takeshita A, Nakamura N, Hirooka Y, Suzuki S, Yoshida M, Nakamura M (September 1988). "Vasodilating effect of the new beta-blocker tilisolol hydrochloride in humans". Arzneimittel-Forschung. 38 (9): 1342–4. PMID 2906248.
- ^ Serradell, MN; Blancafort, P.; Thorpe, PJ; Castaer, J.; N-696. Drugs Fut 1982, 7, 12, 889.
- ^ Hideo Saitama Jp Fukushima, Yoshikuni Kawagoe Saitama Jp Suzuki, DE2631080 (1985 to Nisshin Flour Milling Co., Ltd., Tokio / Tokyo, Jp).
- ^ Lombardino, J. G. (October 1970). "Synthesis of 4‐hydroxy‐2‐methylisocarbostyril‐3‐carboxanilides". Journal of Heterocyclic Chemistry. 7 (5): 1057–1060. doi:10.1002/jhet.5570070509.
- ^ Li Leilei, et al. CN102115459 (2011 to Dihon Pharmaceutical Group Co Ltd).
- ^ Yonezawa K, Sato K, Kobayashi A. High-performance liquid chromatography of a new beta-blocker, 4-[3-(tert.-butylamino)-2-hydroxypropoxy]-N-methylisocarbostyril hydrochloride, in plasma using fluorometric detection. J Chromatogr. 1985 Apr 12;339(1):219-22. doi: 10.1016/s0378-4347(00)84648-4. PMID: 2862154.