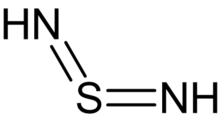
Sulfur diimides are chemical compounds of the formula S(NR)2. Structurally, they are the diimine of sulfur dioxide. The parent member, S(NH)2, is of only theoretical interest. Other derivatives where R is an organic group are stable and useful reagents.
| |
| Names | |
|---|---|
| IUPAC name
diimino-λ4-sulfane
| |
| Other names
2λ4-Diazathia-1,2-diene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| H2N2S | |
| Molar mass | 62.09 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Organic derivatives edit
A particularly stable derivative is di-t-butylsulfurdiimide.[1] It is prepared by reaction of tert-butylamine with sulfur dichloride to give the intermediate "S(N-t-Bu)", which decomposes at 60 °C to give the diimide. A second route to sulfur diimides involve treatment of sulfur tetrafluoride with amines. A third route involves transimidation of disulfonylsulfodiimide:
- S(NSO2Ph)2 + 2 RNH2 → S(NR)2 + 2 PhSO2NH2
N,N'-Bis(methoxycarbonyl)sulfur diimide (MeO2C-N=S=N-CO2Me) is obtained from methyl carbamate.[2]
Structure, bonding, reactions edit
These compounds are related to SO2. They have planar C–N=S=N–C cores with bent C–N=S and N=S=N geometries, and various combinations of E and Z isomers are observed for the two N=S bonds.[3]
Sulfur diimides are electrophilic. They undergo Diels–Alder reactions with dienes.[1] Organolithium reagents attack at the sulfur to give the corresponding nitrogen anion:
- R'Li + S(NR)2 → R'S(NR)(NRLi)
The triimido analogues of sulfite can be generated by treating the sulfur diimides with a metal amide:[4]
- 4 LiNHBu-t + 2 S(NBu-t)2 → 2 Li2S(NBu-t)3 + 2 t-BuNH2
See also edit
- Carbodiimide - the carbon analogue
- Disulfur dinitride
- Bis(trimethylsilyl)sulfur diimide
References edit
- ^ a b Kresze, G.; Wucherpfennig, W., "Organic synthesis with imides of sulfur dioxide", Angew. Chem. Int. Ed. Engl. 1967, volume 6, 149-167. doi:10.1002/anie.196701491
- ^ Kresze, Günter; Braxmeier, Hans; Münsterer, Heribert (1987). "Allylcarbamates by the Aza-Ene Reaction: Methyl N-(2-Methyl-2-Butenyl)Carbamate". Organic Syntheses. 65: 159. doi:10.15227/orgsyn.065.0159.
- ^ Lork, Enno; Mews, Ruëdiger; Shakirov, Makhmut M.; Watson, Paul G.; Zibarev, Andrey V. (2002). "The first N-alkyl-N′-polyfluorohetaryl sulfur diimide". Journal of Fluorine Chemistry. 115 (2): 165–168. doi:10.1016/S0022-1139(02)00047-7.
- ^ Fleischer, R.; Stalke, D., "A new route to sulfur polyimido anions S(NR)nm-: reactivity and coordination behavior", Coord. Chem. Rev. 1998, 176, 431-450. doi:10.1016/S0010-8545(98)00130-1