Rubidium selenide is an inorganic compound composed of selenium and rubidium. It is a selenide with a chemical formula of Rb2Se. Rubidium selenide is used together with caesium selenide in photovoltaic cells.[5]
 Rb+: __ Se2-: __
| |
| Names | |
|---|---|
| IUPAC name
Rubidium selenide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.045.847 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Rb2Se | |
| Molar mass | 249.89 |
| Appearance | colourless, highly hygroscopic crystals[1] |
| Density | 2.912 g/cm3[2] 3.16 g/cm3[3] |
| Melting point | 733 °C[2] |
| hydrolyses[4] | |
| Solubility in other solvents | soluble in ethanol and glycerin |
| Structure | |
| cubic: inverse fluorite structure | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
toxic |
| GHS labelling: | |
  
| |
| H301, H331, H373, H410 | |
| Related compounds | |
Other anions
|
rubidium oxide, rubidium sulfide, rubidium telluride, rubidium polonide |
Other cations
|
lithium selenide, sodium selenide, caesium selenide, francium selenide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation
editRubidium selenide can be prepared by reacting mercury selenide and metallic rubidium.[6] The elements can be synthesized in liquid ammonia.[7]
Hydrogen selenide can also be dissolved in an aqueous solution of rubidium hydroxide to eventually form rubidium selenide.[8] This method is similar to the method for preparing rubidium sulfide, because they are both chalcogenide compounds.
- RbOH + H2Se → RbHSe + H2O
- RbHSe + RbOH → Rb2Se + H2O
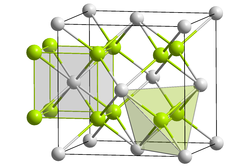
Crystal structure
editRubidium selenide has cubic crystal structure, which belongs to the antifluorite structure, and the space group is and the lattice parameters are a=801.0 pm, per unit. The unit cell has 4 units.[1]
References
edit- ^ a b Jean D'Ans, Ellen Lax: Taschenbuch für Chemiker und Physiker. 3. Elemente, anorganische Verbindungen und Materialien, Minerale, Band 3. 4. Auflage, Springer, 1997, ISBN 978-3-5406-0035-0, S. 692 ([1], p. 692, at Google Books).
- ^ a b Dale L. Perry, Sidney L. Phillips: Handbook of inorganic compounds. CRC Press, 1995, ISBN 978-0-8493-8671-8, S. 336 ([2], p. 336, at Google Books).
- ^ Sommer, Helmut; Hoppe, Rudolf (February 1977). "Die Kristallstruktur von Cs2S. mit einer Bemerkung über Cs2Se, Cs2Te, Rb2Se und Rb2Te" [The crystal structure of cesium sulfide and a remark about cesium selenide, cesium telluride, rubidium selenide, and rubidium telluride]. Zeitschrift für anorganische und allgemeine Chemie (in German). 429 (1): 118–130. doi:10.1002/zaac.19774290116.
- ^ Rubidium selenide at AlfaAesar, accessed on Dienstag, 29. Juni 2010 (PDF) (JavaScript required).[dead link]
- ^ Solid State Technology. Vol. 4. Cowan Publishing Corporation. 1961. p. 34.
- ^ Bergmann, Alfred (1937-03-13). "Über die Darstellung und Eigenschaften von Caesium-und Rubidium-Sulfid, Selenid und Tellurid". Zeitschrift für anorganische und allgemeine Chemie. 231 (3): 269–280. doi:10.1002/zaac.19372310306.
- ^ Mellor, Joseph William (1963). A Comprehensive Treatise on Inorganic and Theoretical Chemistry. Longmans, Green. p. 2178.
- ^ R. Abegg, F. Auerbach: 'Handbuch der anorganischen Chemie'. Verlag S. Hirzel, Bd. 2, 1908. S. 430.Volltext