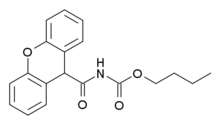
Ro67-4853 is a drug used in scientific research, which acts as a selective positive allosteric modulator for the metabotropic glutamate receptor subtype mGluR1.[1][2][3] It was derived by modification of the simpler compound Ro01-6128, and has itself subsequently been used as a lead compound to develop a range of potent and selective mGluR1 positive modulators.[4][5]
 | |
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H19NO4 |
| Molar mass | 325.364 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
See also
editReferences
edit- ^ Knoflach F, Mutel V, Jolidon S, Kew JN, Malherbe P, Vieira E, Wichmann J, Kemp JA (November 2001). "Positive allosteric modulators of metabotropic glutamate 1 receptor: characterization, mechanism of action, and binding site". Proceedings of the National Academy of Sciences of the United States of America. 98 (23): 13402–7. Bibcode:2001PNAS...9813402K. doi:10.1073/pnas.231358298. PMC 60883. PMID 11606768.
- ^ Hemstapat K, de Paulis T, Chen Y, Brady AE, Grover VK, Alagille D, Tamagnan GD, Conn PJ (August 2006). "A novel class of positive allosteric modulators of metabotropic glutamate receptor subtype 1 interact with a site distinct from that of negative allosteric modulators" (PDF). Molecular Pharmacology. 70 (2): 616–626. doi:10.1124/mol.105.021857. PMID 16645124. S2CID 2719603. Archived from the original (PDF) on 2019-02-22.
- ^ Sheffler DJ, Conn PJ (September 2008). "Allosteric potentiators of metabotropic glutamate receptor subtype 1a differentially modulate independent signaling pathways in baby hamster kidney cells". Neuropharmacology. 55 (4): 419–27. doi:10.1016/j.neuropharm.2008.06.047. PMC 2600811. PMID 18625258.
- ^ Vieira E, Huwyler J, Jolidon S, Knoflach F, Mutel V, Wichmann J (October 2005). "9H-Xanthene-9-carboxylic acid [1,2,4]oxadiazol-3-yl- and (2H-tetrazol-5-yl)-amides as potent, orally available mGlu1 receptor enhancers". Bioorganic & Medicinal Chemistry Letters. 15 (20): 4628–31. doi:10.1016/j.bmcl.2005.05.135. PMID 16099654.
- ^ Vieira E, Huwyler J, Jolidon S, Knoflach F, Mutel V, Wichmann J (March 2009). "Fluorinated 9H-xanthene-9-carboxylic acid oxazol-2-yl-amides as potent, orally available mGlu1 receptor enhancers". Bioorganic & Medicinal Chemistry Letters. 19 (6): 1666–9. doi:10.1016/j.bmcl.2009.01.108. PMID 19233648.