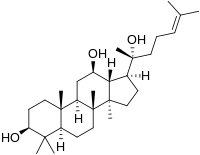
Protopanaxadiol (PPD) is an organic compound that is an aglycone of ginsenosides, a group of steroid glycosides. It is a dammarane-type tetracyclic terpene sapogenin found in ginseng (Panax ginseng) and in notoginseng (Panax pseudoginseng).[1][2]
| |
| Names | |
|---|---|
| IUPAC name
Dammar-24-ene-3β,12β,20-triol
| |
| Systematic IUPAC name
(1S,3aR,3bR,5aR,7S,9aR,9bR,11R,11aR)-1-[(2S)-2-Hydroxy-6-methylhept-5-en-2-yl]-3a,3b,6,6,9a-pentamethylhexadecahydro-1H-cyclopenta[a]phenanthene-7,11-diol | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | PPD |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C30H52O3 | |
| Molar mass | 460.743 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
The health effect of protopanaxadiol inside the human body is still unclear. One study suggests it has rapid, non-genomic effects on endothelial cells, binding to the glucocorticoid and oestrogen beta receptors. The study also showed an increase in intracellular calcium ion concentration.[3]
See also
editReferences
edit- ^ Shibata, S.; Tanaka, O.; Sado, M.; Tsushima, S. (1963). "The genuine sapogenin of ginseng". Tetrahedron Letters. 4 (12): 795–800. doi:10.1016/S0040-4039(01)90718-X.
- ^ Tanaka, O.; Nagai, M.; Shibata, S. (1964). "Stereochemistry of protopanaxadiol, a genuine sapogenin of ginseng". Tetrahedron Letters. 5 (33–34): 2291–7. doi:10.1016/S0040-4039(00)71705-9.
- ^ Leung; et al. (2009). "Protopanaxadiol and protopanaxatriol bind to glucocorticoid and oestrogen receptors in endothelial cells". British Journal of Pharmacology. 156 (4): 626–637. doi:10.1111/j.1476-5381.2008.00066.x. PMC 2697710. PMID 19226254.