Profenofos is an organophosphate insecticide. It is a liquid with a pale yellow to amber color and a garlic-like odor.[1] It was first registered in the United States in 1982.[3]: 1 As of 2015, it was not approved in the European Union.[4]
| |
| Names | |
|---|---|
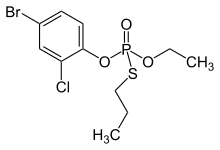
| Preferred IUPAC name
O-(4-Bromo-2-chlorophenyl) O-ethyl S-propyl phosphorothioate | |
| Other names
Phosphorothioic acid, O-(4-bromo-2-chlorophenyl)O-ethylS-propyl ester
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.050.215 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H15BrClO3PS | |
| Molar mass | 373.63 g·mol−1 |
| Appearance | Pale yellow to amber liquid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Uses
editProfenofos can be used on a variety of crops including cotton and vegetables such as maize, potato, soybean, and sugar beet.[5]: 404 In the United States it is used exclusively on cotton and is primarily used against lepidopteran insects.[3]: 1
Mixed with phoxim, cypermethrin, beta-cypermethrin imidacloprid and deltamethrin, profenofos can be used against Cotton MealyBug, cabbage caterpillar, Plutella xylostella and asparagus caterpillars, as well as against wheat and cabbage aphids.[citation needed]
Mechanism of action
editLike other organophosphates, the profenofos mechanism of action is via the inhibition of the acetylcholinesterase enzyme. Although it is used in the form of a racemate, the S(-) isomer is a more potent inhibitor.[5]: 404
Synthesis
editProfenofos can be synthesized by reacting phosphorus oxychloride with sodium ethoxide and sodium 1-propanethiolate, followed by treatment with 4-bromo-2-chlorophenol.[6]: 332
Toxicity
editA 2007 World Health Organization report found no adverse effects to workers of routine exposure to profenofos and no teratogenicity or carcinogenicity.[5]: 435–8
Based on a study of patients poisoned with profenofos and its close chemical relative prothiofos, the compound has been described as "of moderately severe toxicity", causing respiratory failure. Differences in chemical structure that distinguish these two compounds from more common organophosphate pesticides - namely, the presence of the S-alkyl group on the phosphorus atom where most OP compounds possess a methoxy or ethoxy group - underlie differences in their behavior as acetylcholinesterase enzyme inhibitors compared to the rest of the OP class.[7]
In one study of a patient who died of profenofos poisoning, the major metabolites of profenofos were identified as des-S-propylated profenofos, two isomers of despropylated propenofos, and desethylated propenofos.[8] A downstream, nontoxic metabolite, 4-bromo-2-chlorophenol, has been proposed as biomarker for exposure.[9]
Environmental effects
editA United States Environmental Protection Agency report identified profenofos as toxic to birds, small mammals, bees, fish, and aquatic invertebrates, noting several fish kill incidents in which profenofos exposure, primarily due to runoff, was implicated as a probable cause.[3]: 2–3
References
edit- ^ a b CID 38779 from PubChem
- ^ Profenofos in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD) (retrieved 2015)
- ^ a b c "Reregistration Eligibility Decision for Profenofos" (PDF). United States Environmental Protection Agency. US Environmental Protection Agency Office of Pesticide Programs. Retrieved 18 July 2015.
- ^ "EU Pesticides database". European Commission. Retrieved 18 July 2015.
- ^ a b c Pesticide residues in food--2007: toxicological evaluations. Geneva: World Health Organization. 2009. ISBN 9789241665230.
- ^ Braun, David B. (1995). Pharmaceutical manufacturers an international directory. Park Ridge, N.J., U.S.A.: Noyes Publications. ISBN 9780815518532.
- ^ Eddleston, M; Worek, F; Eyer, P; Thiermann, H; Von Meyer, L; Jeganathan, K; Sheriff, MH; Dawson, AH; Buckley, NA (November 2009). "Poisoning with the S-Alkyl organophosphorus insecticides profenofos and prothiofos". QJM. 102 (11): 785–92. doi:10.1093/qjmed/hcp119. PMC 2766103. PMID 19737786.
- ^ Gotoh, M; Sakata, M; Endo, T; Hayashi, H; Seno, H; Suzuki, O (15 February 2001). "Profenofos metabolites in human poisoning". Forensic Science International. 116 (2–3): 221–6. doi:10.1016/s0379-0738(00)00377-7. PMID 11182275.
- ^ Dadson, OA; Ellison, CA; Singleton, ST; Chi, LH; McGarrigle, BP; Lein, PJ; Farahat, FM; Farahat, T; Olson, JR (5 April 2013). "Metabolism of profenofos to 4-bromo-2-chlorophenol, a specific and sensitive exposure biomarker". Toxicology. 306: 35–9. doi:10.1016/j.tox.2013.01.023. PMC 4751995. PMID 23415833.