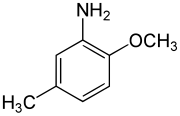
para-Cresidine is an organic compound with the formula CH3OC6H3(CH3)NH2. It is a white solid that is soluble in organic solvents. The compound features both amine and methoxy functional groups. It is used as an intermediate in preparation of dyes and pigments.
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methoxy-5-methylaniline | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.004.018 |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H11NO | |
| Molar mass | 137.179 |
| Appearance | White crystals |
| Melting point | 51.5 °C (124.7 °F; 324.6 K) |
| Boiling point | 235 °C (455 °F; 508 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Synthesis and reactions
editThe compound is obtained in several steps from 4-chlorotoluene. Nitration gives mainly 3-nitro-4-chlorotoluene, which reacts with methoxide sources to give 4-methoxy-2-nitrotoluene. Reduction of this nitro compound affords the aniline.[1]
Sulfonation with oleum gives 4-amino-5-methoxy-2-methylbenzenesulfonic acid. This sulfonic acid is a precursor to allura red AC, a red food coloring.[1]
References
edit- ^ a b P. F. Vogt, J. J. Gerulis, "Amines, Aromatic" in Ullmann’s Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a02_037
External links
edit- International Chemical Safety Card, Center for Disease Control