Niludipine is a calcium channel blocker of the dihydropyridine class. It is a vasodilator that acts upon the coronary arteries of the heart-lung. It was found to produce a calcium antagonistic effect on the smooth muscle of hearts of canines and guinea pigs inhibiting myocardial oxidative metabolism.[1]
| |
| Names | |
|---|---|
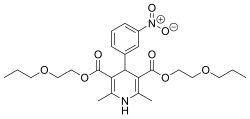
| Preferred IUPAC name
Bis(2-propoxyethyl) 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate | |
| Other names
2,6-Dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylic acid bis(2-propoxyethyl) ester
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.041.003 |
| EC Number |
|
| MeSH | C019497 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C25H34N2O8 | |
| Molar mass | 490.553 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |