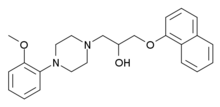
Naftopidil (INN, marketed under the brand name Flivas) is a drug used in benign prostatic hypertrophy which acts as a selective α1-adrenergic receptor antagonist or alpha-1 blocker.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Flivas |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.220.557 |
| Chemical and physical data | |
| Formula | C24H28N2O3 |
| Molar mass | 392.499 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Synthesis
editThe reaction of 1-Naphthol [90-15-3] (1) with Epichlorohydrin [106-89-8] (2) in the presence of alkali led to 2-[(1-Naphthyloxy)Methyl]Oxirane [2461-42-9] (3). Further addition of 1-(2-Methoxyphenyl)Piperazine [35386-24-4] (4) completed the synthesis of naftopidil (5).
See also
editReferences
edit- ^ Sakai H, Igawa T, Onita T, Furukawa M, Hakariya T, Hayashi M, Matsuya F, Shida Y, Nishimura N, Yogi Y, Tsurusaki T, Takehara K, Nomata K, Shiraishi K, Shono T, Aoki D, Kanetake H (2011). "Efficacy of naftopidil in patients with overactive bladder associated with benign prostatic hyperplasia: prospective randomized controlled study to compare differences in efficacy between morning and evening medication". Hinyokika Kiyo. 57 (1): 7–13. PMID 21304253.
- ^ Prous, J., Castañer, J. (1987). "Naftopidil". Drugs of the Future. 12 (1): 31. doi:10.1358/dof.1987.012.01.51182.
- ^ Ernst-Christian Witte, Kurt Stach, Max Thiel, Gisbert Sponer, Egon Roesch, U.S. patent 3,997,666 (1976 to Boehringer Mannheim G.M.B.H.); CA, 83, 179122e
- ^ Shivani, Pujala B, Chakraborti AK. Zinc(II) perchlorate hexahydrate catalyzed opening of epoxide ring by amines: applications to synthesis of (RS)/(R)-propranolols and (RS)/(R)/(S)-naftopidils. J Org Chem. 2007 May 11;72(10):3713-22. doi: 10.1021/jo062674j. Epub 2007 Apr 6. PMID: 17411096.