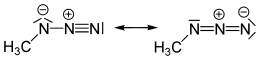
Methyl azide is an organic compound with the formula CH3N3. It is a white solid and it is the simplest organic azide.
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Azidomethane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CH3N3 | |||
| Molar mass | 57.056 g·mol−1 | ||
| Appearance | white powder | ||
| Boiling point | 20–21 °C (68–70 °F; 293–294 K) | ||
| slightly soluble | |||
| Solubility | alkane, ether | ||
| Explosive data | |||
| Shock sensitivity | High | ||
| Friction sensitivity | High | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Highly explosive | ||
| Related compounds | |||
Related compounds
|
Hydrazoic acid, Chlorine azide, Ethyl azide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Preparation and properties
editMethyl azide can be prepared by the methylation of sodium azide, for instance with dimethyl sulfate in alkaline solution, followed by passing through a tube of anhydrous calcium chloride or sodium hydroxide to remove contaminating hydrazoic acid.[1] The first synthesis was reported in 1905.[2]
Decomposition to a nitrene is a first-order reaction:
- CH3N3 → CH3N + N2
The product, like its notional tautomer methanimine, polymerizes at room temperature.[3]
Methyl azide might be a potential precursor in the synthesis of prebiotic molecules via nonequilibrium reactions on interstellar ices initiated by energetic galactic cosmic rays (GCR) and photons.[4]
Safety precautions
editMethyl azide is stable at ambient temperature but may explode when heated. Presence of mercury increases the sensitivity to shock and spark. It is incompatible with methanol and dimethyl malonate.[5] When heated to decomposition, it emits toxic fumes of NO
x.[citation needed] It can be stored indefinitely in the dark at −80 °C.[1]
References
edit- ^ a b Chae, Junghyun (2008-03-14), "Methyl Azide", in John Wiley & Sons, Ltd (ed.), Encyclopedia of Reagents for Organic Synthesis, Chichester, UK: John Wiley & Sons, Ltd, pp. rn00795, doi:10.1002/047084289x.rn00795, ISBN 978-0-471-93623-7
- ^ Dimroth, O.; Wislicenus, W. (1905). "Ueber das Methylazid". Berichte der Deutschen Chemischen Gesellschaft. 38 (2): 1573–1576. doi:10.1002/cber.19050380254.
- ^ O'Dell, M. S.; Darwent, B. (1970). "Thermal decomposition of methyl azide". Canadian Journal of Chemistry. 48 (7): 1140–1147. doi:10.1139/v70-187.
- ^ Quinto-Hernandez, A.; Wodtke, A. M.; Bennett, C. J.; Kim, Y. S.; Kaiser, R. I. (2011). "On the Interaction of Methyl Azide (CH3N3) Ices with Ionizing Radiation: Formation of Methanimine (CH2NH), Hydrogen Cyanide (HCN), and Hydrogen Isocyanide (HNC)". The Journal of Physical Chemistry A. 115 (3): 250–264. doi:10.1021/jp103028v. PMID 21162584.
- ^ Urben, P. G., ed. (2006). Bretherick's Handbook of Reactive Chemical Hazards (7th ed.). Elsevier. ISBN 9780123725639.
External links
edit- Graner, G.; Hirota, E.; Iijima, T.; Kuchitsu, K.; Ramsay, D. A.; Vogt, J.; Vogt, N. (1999). "CH3N3 Methyl azide". In Kuchitsu, K. (ed.). Group II Molecules and Radicals: Numerical Data and Functional Relationships in Science and Technology. Landolt-Börnstein - Group II Molecules and Radicals. Vol. 25 B. p. 1. doi:10.1007/10653318_320. ISBN 3-540-63645-5.
- "Methyl azide". NIST Webbook. National Institute for Standards and Technology.

