Malonyl-CoA is a coenzyme A derivative of malonic acid.
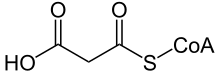
| |
| Names | |
|---|---|
| Preferred IUPAC name
(9R)-1-[(2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]-3,5,9-trihydroxy-3,5,10,14,19-pentaoxo-8,8-dimethyl-2,4,6-trioxa-18-thia-11,15-diaza-3λ5,5λ5-diphosphahenicosan-21-oic acid | |
| Identifiers | |
| |
| ChemSpider | |
| ECHA InfoCard | 100.007.596 |
| MeSH | Malonyl+CoA |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C24H38N7O19P3S | |
| Molar mass | 853.582 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Functions
editIt plays a key role in chain elongation in fatty acid biosynthesis and polyketide biosynthesis.
Cytosolic fatty acid biosynthesis
editMalonyl-CoA provides 2-carbon units to fatty acids and commits them to fatty acid chain synthesis.
Malonyl-CoA is formed by carboxylating acetyl-CoA using the enzyme acetyl-CoA carboxylase. One molecule of acetyl-CoA joins with a molecule of bicarbonate,[1] requiring energy rendered from ATP.
Malonyl-CoA is utilised in fatty acid biosynthesis by the enzyme malonyl coenzyme A:acyl carrier protein transacylase (MCAT). MCAT serves to transfer malonate from malonyl-CoA to the terminal thiol of holo-acyl carrier protein (ACP).
Mitochondrial fatty acid synthesis
editMalonyl-CoA is formed in the first step of mitochondrial fatty acid synthesis (mtFASII) from malonic acid by malonyl-CoA synthetase (ACSF3).[2][3]
Polyketide biosynthesis
editMCAT is also involved in bacterial polyketide biosynthesis. The enzyme MCAT together with an acyl carrier protein (ACP), and a polyketide synthase (PKS) and chain-length factor heterodimer, constitutes the minimal PKS of type II polyketides.
Regulation
editMalonyl-CoA is a highly regulated molecule in fatty acid synthesis; as such, it inhibits the rate-limiting step in beta-oxidation of fatty acids. Malonyl-CoA inhibits fatty acids from associating with carnitine by regulating the enzyme carnitine acyltransferase, thereby preventing them from entering the mitochondria, where fatty acid oxidation and degradation occur.
Related diseases
editMalonyl-CoA plays a special role in the mitochondrial clearance of toxic malonic acid in the metabolic disorder combined malonic and methylmalonic aciduria (CMAMMA).[4] In CMAMMA due to ACSF3, malonyl-CoA synthetase is decreased, which can generate malonyl-CoA from malonic acid, which can then be converted to acetyl-CoA by malonyl-CoA decarboxylase.[2][4] In contrast, in CMAMMA due to malonyl-CoA decarboxylase deficiency, malonyl-CoA decarboxylase is decreased, which converts malonyl-CoA to acetyl-CoA.[4]
See also
editReferences
edit- ^ Nelson D, Cox M (2008). Lehninger principles of biochemistry (5th ed.). p. 806.
- ^ a b Witkowski, Andrzej; Thweatt, Jennifer; Smith, Stuart (September 2011). "Mammalian ACSF3 Protein Is a Malonyl-CoA Synthetase That Supplies the Chain Extender Units for Mitochondrial Fatty Acid Synthesis". Journal of Biological Chemistry. 286 (39): 33729–33736. doi:10.1074/jbc.M111.291591. ISSN 0021-9258. PMC 3190830. PMID 21846720.
- ^ Bowman, Caitlyn E.; Rodriguez, Susana; Selen Alpergin, Ebru S.; Acoba, Michelle G.; Zhao, Liang; Hartung, Thomas; Claypool, Steven M.; Watkins, Paul A.; Wolfgang, Michael J. (2017). "The Mammalian Malonyl-CoA Synthetase ACSF3 Is Required for Mitochondrial Protein Malonylation and Metabolic Efficiency". Cell Chemical Biology. 24 (6): 673–684.e4. doi:10.1016/j.chembiol.2017.04.009. PMC 5482780. PMID 28479296.
- ^ a b c Bowman, Caitlyn E.; Wolfgang, Michael J. (January 2019). "Role of the malonyl-CoA synthetase ACSF3 in mitochondrial metabolism". Advances in Biological Regulation. 71: 34–40. doi:10.1016/j.jbior.2018.09.002. PMC 6347522. PMID 30201289.