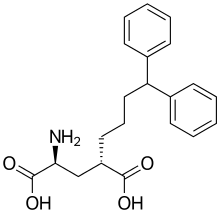
LY-307,452 is a drug used in neuroscience research, which was among the first compounds found that acts as a selective antagonist for the group II metabotropic glutamate receptors (mGluR2/3),[1] and was useful in early studies of this receptor family,[2][3] although it has largely been replaced by newer drugs such as LY-341,495. Its molecular formula is C21H25NO4[4]
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H25NO4 |
| Molar mass | 355.434 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
References
edit- ^ Wermuth CG, Mann A, Schoenfelder A, Wright RA, Johnson BG, Burnett JP, Mayne NG, Schoepp DD (February 1996). "(2S,4S)-2-amino-4-(4,4-diphenylbut-1-yl)-pentane-1,5-dioic acid: a potent and selective antagonist for metabotropic glutamate receptors negatively linked to adenylate cyclase". Journal of Medicinal Chemistry. 39 (4): 814–6. doi:10.1021/jm9508144. PMID 8632404.
- ^ Li XC, Beart PM, Monn JA, Jones NM, Widdop RE (October 1999). "Type I and II metabotropic glutamate receptor agonists and antagonists evoke cardiovascular effects after intrathecal administration in conscious rats". British Journal of Pharmacology. 128 (3): 823–9. doi:10.1038/sj.bjp.0702850. PMC 1571690. PMID 10516668.
- ^ Sung KW, Choi S, Lovinger DM (November 2001). "Activation of group I mGluRs is necessary for induction of long-term depression at striatal synapses". Journal of Neurophysiology. 86 (5): 2405–12. doi:10.1152/jn.2001.86.5.2405. PMID 11698530. S2CID 15299425.
- ^ "LY-307,452 | C21H25NO4 | ChemSpider".