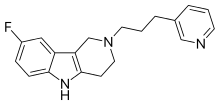
Gevotroline (WY-47,384) is an atypical antipsychotic with a tricyclic structure which was under development for the treatment of schizophrenia by Wyeth-Ayerst.[1][2][3] It acts as a balanced, modest affinity D2 and 5-HT2 receptor antagonist and also possesses high affinity for the sigma receptor.[2][4][5][6] It was well tolerated and showed efficacy in phase II clinical trials but was never marketed.[2][3]
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H20FN3 |
| Molar mass | 309.388 g·mol−1 |
| 3D model (JSmol) | |
| |
See also
editReferences
edit- ^ Triggle DJ (1996). Dictionary of Pharmacological Agents. Boca Raton: Chapman & Hall/CRC. ISBN 0-412-46630-9.
- ^ a b c Abou-Gharbia M, Moyer JA (1990). Bristol JA (ed.). "Novel Antipyschotic Agents". Annual Reports in Medicinal Chemistry. 25. Boston: Academic Press: 1–10. doi:10.1016/S0065-7743(08)61577-8. ISBN 0-12-040525-3.
- ^ a b Jackson DM, Mohell N (1996). "A Review of the Pharmacology of New Antipsychotic Drugs". In Stone TW (ed.). CNS neurotransmitters and neuromodulators: dopamine. Boca Raton: CRC Press. ISBN 0-8493-7632-7.
- ^ Snyder SH, Largent BL (1989). "Receptor mechanisms in antipsychotic drug action: focus on sigma receptors". The Journal of Neuropsychiatry and Clinical Neurosciences. 1 (1): 7–15. doi:10.1176/jnp.1.1.7. PMID 2577720.
- ^ Matheson GK, Guthrie D, Bauer C, Knowles A, White G, Ruston C (January 1991). "Sigma receptor ligands alter concentrations of corticosterone in plasma in the rat". Neuropharmacology. 30 (1): 79–87. doi:10.1016/0028-3908(91)90046-E. PMID 1675451. S2CID 29702968.
- ^ Gudelsky GA, Nash JF (February 1992). "Neuroendocrinological and neurochemical effects of sigma ligands". Neuropharmacology. 31 (2): 157–162. doi:10.1016/0028-3908(92)90026-L. PMID 1348112. S2CID 36585024.