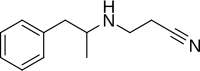
Fenproporex (Perphoxene) (N-2-Cyanoethylamphetamine) (3-(1-phenylpropan-2-ylamino)propanenitrile) (3-[(1-Methyl-2-Phenylethyl)amino]propiononitrile) is a stimulant drug of the phenethylamine and amphetamine chemical classes that was developed in the 1960s. It is used as an appetite suppressant for the treatment of obesity.[3]
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth[1] |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | partly converted to amphetamine (30 to 60%)[1] |
| Excretion | urine, mainly as amphetamine, about 5 to 9% unchanged[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.036.752 |
| Chemical and physical data | |
| Formula | C12H16N2 |
| Molar mass | 188.274 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Fenproporex produces amphetamine as a metabolite[4][5] and was withdrawn in many countries following problems with abuse,[6] but it is still prescribed in some countries. It is sometimes combined with benzodiazepines, antidepressants, and other compounds to create a version of the "rainbow diet pill".[7][8][9]
Fenproporex has never been approved by the US Food and Drug Administration (FDA) for sale in the US due to lack of efficacy and safety data. However, in March 2009, the FDA warned consumers that it has been detected as an unlabeled component of diet pills available over the Internet.[10] Fenproporex is designated a Schedule IV controlled substance in the US pursuant to the Controlled Substances Act.[11]
Fenproporex is on the list of substances banned by the World Anti-Doping Agency, and any sportsperson testing positive for the substance faces a ban from competition.[12]
References
edit- ^ a b c Seyffart G (1991). Drug dosage in renal insufficiency. Springer. pp. 245–246. ISBN 978-0-7923-0964-2.
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ Warembourg H, Jaillard J. Clinical experimentation with fenproporex in the treatment of obesity. Apropos of 40 cases. (French). Journal de la Faculte de médecine et de pharmacie de l'Universite de Lille. 1968 Mar;13(3):Suppl:273-6.
- ^ Tognoni G, Morselli PL, Garattini S (October 1972). "Amphetamine concentrations in rat brain and human urine after fenproporex administration". European Journal of Pharmacology. 20 (1): 125–6. doi:10.1016/0014-2999(72)90227-0. PMID 4637940.
- ^ Cody JD (December 1993). "Metabolic Precursors to Amphetamine and Methamphetamine". Forensic Science Review. 5 (2): 109–27. PMID 26270078.
- ^ Pélissier-Alicot AL, Piercecchi-Marti MD, Bartoli C, Kuhlmann E, Coiffait PE, Sanvoisin A, et al. (March 2006). "Abusive prescription of psychostimulants: a study of two cases". Journal of Forensic Sciences. 51 (2): 407–10. doi:10.1111/j.1556-4029.2006.00078.x. PMID 16566781. S2CID 39460850.
- ^ Cohen PA (March 2009). "Imported fenproporex-based diet pills from Brazil: a report of two cases". Journal of General Internal Medicine. 24 (3): 430–3. doi:10.1007/s11606-008-0878-4. PMC 2642570. PMID 19096898.
- ^ Cohen PA, McCormick D, Casey C, Dawson GF, Hacker KA (June 2009). "Imported compounded diet pill use among Brazilian women immigrants in the United States". Journal of Immigrant and Minority Health. 11 (3): 229–36. doi:10.1007/s10903-007-9099-x. PMID 18066718. S2CID 8730835.
- ^ Cohen PA, Goday A, Swann JP (September 2012). "The return of rainbow diet pills". American Journal of Public Health. 102 (9): 1676–86. doi:10.2105/AJPH.2012.300655. PMC 3482033. PMID 22813089.
- ^ "Consumer Directed Questions and Answers about FDA's Initiative Against Contaminated Weight Loss Products". FDA/Center for Drug Evaluation and Research. Archived from the original on 17 March 2009.
- ^ "21 CFR 1308.14(e)(4)". U.S. Government Printing Office. Archived from the original on 2011-10-16. Retrieved 2011-04-20.
- ^ The 2009 Prohibited List International Standard Archived February 3, 2009, at the Wayback Machine. World Anti-Doping Agency (2009). Retrieved on 2009-08-18.