This article needs additional citations for verification. (November 2019) |
Ethyl tertiary-butyl ether (ETBE), also known as ethyl tert-butyl ether, is commonly used as an oxygenate gasoline additive in the production of gasoline from crude oil. ETBE offers equal or greater air quality benefits than ethanol, while being technically and logistically less challenging. Unlike ethanol, ETBE does not induce evaporation of gasoline, which is one of the causes of smog, and does not absorb moisture from the atmosphere.
| |

| |
| Names | |
|---|---|
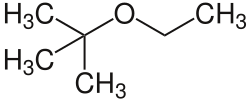
| Preferred IUPAC name
2-Ethoxy-2-methylpropane | |
| Other names
Ethyl tert-butyl ether
Ethyl tertiary butyl ether Ethyl tert-butyl oxide tert-Butyl ethyl ether Ethyl t-butyl ether | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | ETBE |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.010.282 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H14O | |
| Molar mass | 102.18 |
| Appearance | Clear colorless liquid |
| Density | 0.7364 g/cm3 |
| Melting point | −94 °C (−137 °F; 179 K) |
| Boiling point | 69 to 71 °C (156 to 160 °F; 342 to 344 K) |
| 1.2 g/100 g | |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H224, H225, H315, H319, H335, H336 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P370+P378, P403+P233, P403+P235, P405, P501 | |
| Flash point | −19 °C (−2 °F; 254 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Production
editEthyl tert-butyl ether is manufactured industrially by the acidic etherification of isobutylene with ethanol at a temperature of 30–110 °C and a pressure of 0,8–1,3 MPa. The reaction is carried out with an acidic ion-exchange resin as a catalyst.[2]
Suitable reactors are fixed-bed reactors such as tube bundle or circulation reactors in which the reflux can be cooled optionally.[2]
Ethanol, produced by fermentation and distillation, is more expensive than methanol, which is derived from natural gas. Therefore, MTBE, made from methanol is cheaper than ETBE, made from ethanol.
See also
edit- Methyl tert-butyl ether (MTBE)
- tert-Amyl methyl ether (TAME)
- Tetraethyllead (TEL)
- List of gasoline additives
References
edit- ^ Merck Index, 11th Edition, 3732.
- ^ a b Grömping, Matthias; Höper, Frank; Leistner, Jörg; Nierlich, Franz; Peters, Udo; Praefke, Jochen; Rix, Armin; Röttger, Dirk; Santiago Fernandez, Silvia. "Preparing ethyl tertiary butylether from hydrocarbon mixture, useful as fuel additive, comprises reacting isobutene with ethanol, separating the hydrocarbon, reacting separated isobutene with ethanol and separating unconverted hydrocarbon". Google Patents. Evonik Degussa GmbH. Retrieved 5 March 2019.
External links
edit- EC Joint Research Centre ETBE risk assessment report[permanent dead link]
- Directive 98/70/EC of the European Parliament and of the Council of 13 October 1998 relating to the quality of petrol and diesel fuels and amending Council Directive 93/12/EEC
- An assessment of the impact of ethanol-blended petrol on the total NMVOC emission from road transport in selected countries