Eplivanserin (SR-46,349; planned trade name Ciltyri) was an experimental drug for the treatment of insomnia which was being developed by Sanofi Aventis.[1]
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.189.857 |
| Chemical and physical data | |
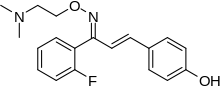
| Formula | C19H21FN2O2 |
| Molar mass | 328.387 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Sanofi Aventis announced in December 2009 that it was withdrawing its application for approval of eplivanserin from both the U.S. Food and Drug Administration and the European Medicines Agency.[2]
Mechanism of action
editEplivanserin is an inverse agonist on the serotonin receptor subtype 5-HT2A. In contrast to older sedating drugs acting on 5-HT2A receptors (e.g., mirtazapine, clozapine, risperidone), eplivanserin has practically no affinity to dopamine, histamine and adrenergic receptors.[3]
Study results
editIn a placebo controlled Phase II clinical trial with 351 subjects, eplivanserin reduced the sleep latency by 39 minutes (versus 26 minutes under placebo).[3]
Synthesis
editThe condensation between 2'-Fluoroacetophenone [445-27-2] (5) & 4-hydroxybenzaldehyde [123-08-0] (6) give a chalcone intermediate (also an enone), i.e. CID:53982926 (7).
(2-chloroethyl)dimethylamine (CDMA) & acetone oxime are reacted together to give dimethylaminoacetoxime (DMA acetoxime), CID:16641114 (3).
Convergent synthesis gives the product as a mixture of isomers.
See also
editReferences
edit- ^ "Future Treatments for Depression, Anxiety, Sleep Disorders, Psychosis, and ADHD". Neurotransmitter.net.
- ^ Spencer M, Berton E (21 December 2009). "Sanofi-Aventis Discontinues Eplivanserin For Insomnia". Dow Jones & Co. Archived from the original on 21 July 2011. Retrieved 27 January 2010.
- ^ a b Teegarden BR, Al Shamma H, Xiong Y (2008). "5-HT(2A) inverse-agonists for the treatment of insomnia". Current Topics in Medicinal Chemistry. 8 (11): 969–76. doi:10.2174/156802608784936700. PMID 18673166.
- ^ Eric Garcia, Christian Hoff, U.S. patent 20,120,022,292 (2012 to Sanofi-Aventis).