 | Draft article not currently submitted for review.
This is a draft Articles for creation (AfC) submission. It is not currently pending review. While there are no deadlines, abandoned drafts may be deleted after six months. To edit the draft click on the "Edit" tab at the top of the window. To be accepted, a draft should:
It is strongly discouraged to write about yourself, your business or employer. If you do so, you must declare it. Where to get help
How to improve a draft
You can also browse Wikipedia:Featured articles and Wikipedia:Good articles to find examples of Wikipedia's best writing on topics similar to your proposed article. Improving your odds of a speedy review To improve your odds of a faster review, tag your draft with relevant WikiProject tags using the button below. This will let reviewers know a new draft has been submitted in their area of interest. For instance, if you wrote about a female astronomer, you would want to add the Biography, Astronomy, and Women scientists tags. Editor resources
Last edited by Ldm1954 (talk | contribs) 3 seconds ago. (Update) |
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these template messages)
|

Photo-ATRP (photoinduced atom transfer radical polymerization)[1] is a form of polymerization which was developed based on atom transfer radical polymerization (ATRP) to further optimize the ATRP process. By introducing a photoreductant to replace traditional reductants, the ATRP reaction can occur in the presence of oxygen, as the photoreductant consumes oxygen during the reaction, enabling ATRP under aerobic conditions. Consequently, Photo-ATRP allows for control over the polymerization process through the on/off switching of light and expands the applicable conditions of the reaction to include aerobic environments.
There are two examples of Photo-ATRP: Photo-ATRP with methylene blue and Photo-ATRP with zinc porphyrin.
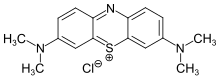
Photo-ATRP with methylene blue
editMatyjaszewski group introduced the first example of a fully oxygen-tolerant red/NIR-light-mediated photoinduced atom transfer radical polymerization (photo-ATRP) that operates in a high-throughput manner under biologically relevant conditions.[2] This method utilizes commercially available methylene blue (MB+) as the photoredox catalyst (PC) and a [X−CuII/TPMA]+ complex (with TPMA being tris(2-pyridylmethyl)amine) as the deactivator.
This method is significant because it:
- Utilizes methylene blue (MB+) as a commercially available photocatalyst and a copper complex with tris(2-pyridylmethyl)amine (TPMA) as the deactivator.
- Demonstrates a reductive quenching cycle involving MB+ in the presence of TPMA, which sustains polymerization by regenerating the Cu(I)/TPMA activator.
- Achieves high monomer conversions (>90%) in less than 60 minutes, even in small volumes (50−250 μL).
- Allows for the high-throughput synthesis of a diverse library of well-defined polymers and DNA−polymer bioconjugates with narrow molecular weight distributions (Đ < 1.30) in an open-air 96-well plate.
- Exhibits excellent biocompatibility during polymerization in the presence of cells, expanding potential applications.
The broad absorption spectrum of MB+ enables ATRP to be triggered under a range of light conditions from UV to NIR (395−730 nm), paving the way for the integration of orthogonal photoinduced reactions. This advancement in polymer science offers a more efficient, productive, and cost-effective method for synthesizing polymers with complex architectures under environmentally benign conditions.
Photo-ATRP with zinc porphyrin
editIn another work, Matyjaszewski group introduced a dual photoredox catalytic system that mediates photoinduced Atom Transfer Radical Polymerization (ATRP) under red-light irradiation.[3] This system is designed to overcome the limitations associated with the use of UV light in traditional photochemical ATRP processes.
The key components of this system include:
- A copper (Cu) catalyst that controls the polymerization through ATRP equilibrium.
- A photocatalyst, such as zinc(II) tetraphenylporphine or zinc(II) phthalocyanine, which generates the activator Cu(I) species when exposed to red light.
One of the notable achievements of this system is its oxygen tolerance. Unlike previous methods that required deoxygenation, this system can consume oxygen in the photoredox reactions, allowing for well-controlled polymerizations without the need to remove oxygen.
The integration of photochemical processes in controlled polymerizations has opened new opportunities for synthesizing well-defined polymeric materials. The efficiency of these processes depends on the effective transfer of photon energy to activate or drive polymerization, which can be achieved through direct excitation of polymer chain ends or by using various photocatalytic/photosensitizer systems.
Overall, this study presents a novel approach that enhances the practicality and scope of ATRP by utilizing red light, which is less harmful than UV light, and by simplifying the polymerization process through oxygen tolerance
References
edit- ^ University, Carnegie Mellon. "Photoinitiated ATRP - Matyjaszewski Polymer Group - Carnegie Mellon University". www.cmu.edu. Retrieved 2024-06-07.
- ^ Hu, Xiaolei; Szczepaniak, Grzegorz; Lewandowska-Andralojc, Anna; Jeong, Jaepil; Li, Bingda; Murata, Hironobu; Yin, Rongguan; Jazani, Arman Moini; Das, Subha R.; Matyjaszewski, Krzysztof (2023-11-08). "Red-Light-Driven Atom Transfer Radical Polymerization for High-Throughput Polymer Synthesis in Open Air". Journal of the American Chemical Society. 145 (44): 24315–24327. doi:10.1021/jacs.3c09181. ISSN 0002-7863. PMC 10636753. PMID 37878520.
- ^ Dadashi-Silab, Sajjad; Kim, Khidong; Lorandi, Francesca; Szczepaniak, Grzegorz; Kramer, Stephanie; Peteanu, Linda; Matyjaszewski, Krzysztof (2022-03-15). "Red-Light-Induced, Copper-Catalyzed Atom Transfer Radical Polymerization". ACS Macro Letters. 11 (3): 376–381. doi:10.1021/acsmacrolett.2c00080. ISSN 2161-1653. PMID 35575360.