DHA-paclitaxel (or Taxoprexin) is an investigational drug (from Protarga Inc) made by linking paclitaxel to docosahexaenoic acid (DHA), a fatty acid that is easily taken up by tumor cells; the DHA-paclitaxel “appears not to be cytotoxic until the bond with DHA is cleaved within the cell.”[1] The advantage of DHA-paclitaxel over paclitaxel is DHA-paclitaxel's ability to carry much higher concentrations of paclitaxel to the cells, which are maintained for longer periods in the tumor cells, thus increasing their action. With increased activity, DHA-paclitaxel, also known as Taxoprexin, may have a more successful response in cancer patients than Taxol, and it may be able to treat more types of cancer than Taxol has been able to treat.
| |
| Names | |
|---|---|
| IUPAC name
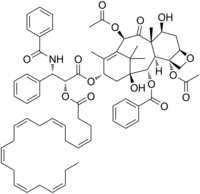
1,7β-Dihydroxy-9-oxo-5β,20-epoxytax-11-ene-2α,4α,10β,13α-tetrayl 4,10-diacetate 13-[(2R,3S)-3-benzamido-2-{[(4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoyl]oxy}-3-phenylpropanoate] 2-benzoate
| |
| Systematic IUPAC name
(2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-4,11-Dihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methano[1]benzoxeto[3,4-a][10]annulene-6,9,12,12b-tetrayl 6,12b-diacetate 9-[(2R,3S)-3-benzamido-2-{[(4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoyl]oxy}-3-phenylpropanoate] 12-benzoate | |
| Other names
Docosahexaenoyl-paclitaxel; Taxoprexin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C69H81NO15 | |
| Molar mass | 1164.399 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Clinical trials
editIn 2007, a phase II clinical trial reported "modest activity in patients with oesophago-gastric cancer".[2]
References
edit- ^ Whelan, Jo (2002). "Targeted taxane therapy for cancer". Drug Discovery Today. 7 (2): 90–92. doi:10.1016/S1359-6446(01)02149-3. PMID 11790612.
- ^ Jones, RJ; Hawkins, RE; Eatock, MM; Ferry, DR; Eskens, FA; Wilke, H; Evans, TR (2008). "A phase II open-label study of DHA-paclitaxel (Taxoprexin) by 2-h intravenous infusion in previously untreated patients with locally advanced or metastatic gastric or oesophageal adenocarcinoma". Cancer Chemotherapy and Pharmacology. 61 (3): 435–41. doi:10.1007/s00280-007-0486-8. PMID 17440725.