Cytochalasin E, a member of the cytochalasin group, is an inhibitor of actin polymerization in blood platelets. It inhibits angiogenesis and tumor growth. Unlike cytochalasin A and cytochalasin B, it does not inhibit glucose transport. Cytochalasin E, however, was noted to decrease glucose absorption in mice around the intestinal tissues by increasing the Km needed for glucose to reach the Vmax, which meant that a higher concentration of glucose was required in its presence to attain Vmax. Since Vmax remained the same according to another study, it is evident that CE is indeed a competitive inhibitor at the intestinal receptor sites for glucose.[2]
| |
| Names | |
|---|---|
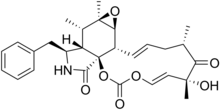
| Preferred IUPAC name
(4E,6R,8S,10E,11aS,11bS,12aR,13S,13aS,14S)-14-Benzyl-6-hydroxy-6,8,12a,13-tetramethyl-9,11a,11b,12a,13,13a,14,15-octahydro-2H-[1,3]dioxacyclotridecino[4,5-d]oxireno[2,3-f]isoindole-2,7,16(6H,8H)-trione | |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.048.018 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C28H33NO7 | |
| Molar mass | 495.572 g·mol−1 |
| Density | 1.309 g/ml |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Toxic |
| GHS labelling: | |
 
| |
| Danger | |
| H300, H310, H330, H361 | |
| P201, P202, P260, P262, P264, P270, P271, P280, P281, P284, P301+P310, P302+P350, P304+P340, P308+P313, P310, P320, P321, P322, P330, P361, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Because of its antiangiogenic effect, cytochalasin E is a potential drug for age-related macular degeneration, a kind of blindness caused by an abnormal proliferation of blood vessels in the eye.[3]
Cytochalasin E was found to be a potent and selective inhibitor of bovine capillary endothelial (BCE) cell proliferation. Cytochalasin E differs from other cytochalasin molecules by having an epoxide, which is required for specificity and potency. Cytochalasin E is a potent antiangiogenic agent that may be useful for treatments of cancer and other pathologic angiogenesis.[4] Cytochalasin E was also found to inhibit autophagy, a process vital in recycling dysfunctional cells and cellular components. Cancer cells thus favor autophagy due to its role in countering metabolic stresses induced by anti-cancer drugs such as bortezomib in order to regenerate healthier cancer cells for continued proliferation and growth. In a study, it was confirmed that when CE was used, fusion of autophagosomes with lysozyme was inhibited and so cell death due to bortezomib, a proteasome inhibitor, was amplified as unnecessary proteins would continue to build up inside cancer cells unable to be further recycled through autophagy, leading to apoptosis.[5]
References
edit- ^ Cytochalasin E from Aspergillus clavatus at Sigma-Aldrich
- ^ Glinsukon, T.; Kongsuktrakoon, B.; Toskulkao, C.; Sophasan, S. (1983-03-01). "Cytochalasin E: inhibition of intestinal glucose absorption in the mouse". Toxicology Letters. 15 (4): 341–348. doi:10.1016/0378-4274(83)90154-6. ISSN 0378-4274. PMID 6836602.
- ^ "Cytochalasin E". 2006-05-19. Archived from the original on 19 May 2006. Retrieved 2022-05-12.
- ^ Udagawa, T.; Yuan, J.; Panigrahy, D.; Chang, Y. H.; Shah, J.; D'Amato, R. J. (2000-08-01). "Cytochalasin E, an epoxide containing Aspergillus-derived fungal metabolite, inhibits angiogenesis and tumor growth". The Journal of Pharmacology and Experimental Therapeutics. 294 (2): 421–427. ISSN 0022-3565. PMID 10900214.
- ^ Takanezawa, Yasukazu; Nakamura, Ryosuke; Kojima, Yuka; Sone, Yuka; Uraguchi, Shimpei; Kiyono, Masako (2018-04-06). "Cytochalasin E increased the sensitivity of human lung cancer A549 cells to bortezomib via inhibition of autophagy". Biochemical and Biophysical Research Communications. 498 (3): 603–608. doi:10.1016/j.bbrc.2018.03.029. ISSN 1090-2104. PMID 29524420.
External pages
editCytochalasin E from Fermentek
Cytochalasin E from Cayman Chemical