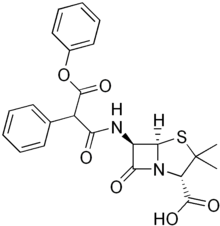
Carfecillin is a beta-lactam antibiotic. It is a phenyl derivative of carbenicillin, acting as a prodrug.[1]
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.043.778 |
| Chemical and physical data | |
| Formula | C23H22N2O6S |
| Molar mass | 454.50 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
References
edit- ^ Basker MJ, Comber KR, Sutherland R, Valler GH (1977). "Carfecillin: antibacterial activity in vitro and in vivo". Chemotherapy. 23 (6): 424–35. doi:10.1159/000222012. PMID 21771.