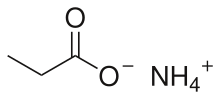
Ammonium propionate or ammonium propanoate is the ammonium salt of propionic acid. It has the chemical formula NH4(C2H5COO).
| |
| Names | |
|---|---|
| IUPAC name
Ammonium propanoate
| |
| Other names
Ammonium propionate
propanoic acid, ammonium salt(1:1) | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.037.715 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H9NO2 | |
| Molar mass | 91.110 g·mol−1 |
| Melting point | 45 °C (113 °F; 318 K) |
| Boiling point | 141.7 °C (287.1 °F; 414.8 K) |
| 1 g/mL | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Irritant |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Reaction
editIt is formed by the reaction of propionic acid and ammonia.
Uses
editIt is used in several products, which include: fertilizers, water treatment chemicals, and plant protection products. It is also used in different areas, such as: manufacturing, forestry, agriculture, and fishing.[1]
It also serves as an antiseptic, antifungal agent, antimould agent, and preservative in feed industry or food industry.[2]
Ammonium propionate also prevents spoilage of cosmetics by preventing bacterial growth.[3]
See also
editReferences
edit- ^ "Ammonium propionate - Substance Information - ECHA". echa.europa.eu. Retrieved 2021-02-22.
- ^ "Ammonium Propionate Properties, Molecular Formula, Applications - WorldOfChemicals". www.worldofchemicals.com. Archived from the original on 2022-02-17. Retrieved 2021-02-22.
- ^ "Ammonium Propionate | Cosmetics Info". cosmeticsinfo.org. Archived from the original on 2017-09-29. Retrieved 2021-02-22.