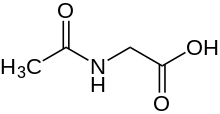
Aceturic acid (N-acetylglycine) is a derivative of the amino acid glycine. The conjugate base of this carboxylic acid is called aceturate, a term used for its esters and salts.
| |
| Names | |
|---|---|
| Preferred IUPAC name
Acetamidoacetic acid | |
| Other names
Acetylglycine
N-Acetylglycine 2-Acetamidoacetic acid Acetylglycocoll | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | AcGly |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.008.036 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H7NO3 | |
| Molar mass | 117.104 g·mol−1 |
| Appearance | White powder or needles |
| Melting point | 206 to 208 °C (403 to 406 °F; 479 to 481 K) |
| 2.7% at 15 °C | |
| Acidity (pKa) | 3.67 (H2O)[1] |
| Related compounds | |
Related compounds
|
N-Acetylglycinamide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation
editAceturic acid can be prepared by warming glycine either with a slight excess of acetic anhydride in benzene,[2] or with an equal molar amount of acetic anhydride in glacial (concentrated) acetic acid.[3]
See also
editReferences
edit- ^ Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. p. 5–88. ISBN 978-1498754286.
- ^ Curtius, Th.; Radenhausen, R. (1895). "Hydrazide und Azide organischer Säuren. X Abhandlung. 35. Ueber Hydrazide substituirter Amidosäuren und das Hydrazid der Fumarsäure". J. Prakt. Chem. 52 (1): 433–454. doi:10.1002/prac.18950520134.
- ^ Dakin, H. D. (1929). "The Condensation of Aromatic Aldehydes with Glycine and Acetylglycine" (PDF). J. Biol. Chem. 82 (2): 439–446. doi:10.1016/S0021-9258(20)78291-8.