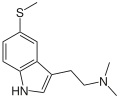
5-MeS-DMT (5-methylthio-N,N-dimethyltryptamine) is a lesser-known psychedelic drug. It is the 5-methylthio analog of dimethyltryptamine (DMT). 5-MeS-DMT was first synthesized by Alexander Shulgin. In his book TiHKAL (Tryptamines I Have Known and Loved), the minimum dosage is listed as 15-30 mg.[1] The duration listed as very short (less than one hour), just like DMT. 5-MeS-DMT produces similar effects to DMT, but weaker. Shulgin describes his feelings while on a low dose of this drug as "pointlessly stoned", although at a higher dose of 20 mg he says it is "quite intense" and suggests that a higher dose still might have full activity.
| |
| Names | |
|---|---|
| Preferred IUPAC name
N,N-Dimethyl-2-[5-(methylsulfanyl)-1H-indol-3-yl]ethan-1-amine | |
| Other names
5-Methylthio-N,N-dimethyltryptamine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C13H18N2S | |
| Molar mass | 234.36 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
5-MeS-DMT has been the subject of only limited clinical testing, with several small behavioral studies in rats indicating that it is a less potent than 5-MeO-DMT or 4-hydroxy-DMT (psilocin) but more effective than either 4-MeO-DMT or 4-MeS-DMT.[2][3][4]
See also
editReferences
edit- ^ 5-MeS-DMT Entry in TIHKAL
- ^ Glennon, R.A.; Young, R.; Benington, F.; Morin, R.D. (February 1982). "Hallucinogens as discriminative stimuli: A comparison of 4-OMe and 5-OMe DMT with their methylthio counterparts". Life Sciences. 30 (5): 465–467. doi:10.1016/0024-3205(82)90463-5. PMID 6801410.
- ^ Kline TB; Benington F; Morin RD; Beaton JM (August 1982). "Structure-activity relationships in potentially hallucinogenic N,N-dialkyltryptamines substituted in the benzene moiety". Journal of Medicinal Chemistry. 25 (8): 908–13. doi:10.1021/jm00350a005. PMID 7120280.
- ^ Kline TB; Benington F; Morin RD; Beaton JM; Glennon RA; Domelsmith LN; Houk KN; Rozeboom MD (November 1982). "Structure-activity relationships for hallucinogenic N,N-dialkyltryptamines: photoelectron spectra and serotonin receptor affinities of methylthio and methylenedioxy derivatives". Journal of Medicinal Chemistry. 25 (11): 1381–3. doi:10.1021/jm00353a021. PMID 6815326.