βOH-2C-B (also known as beta-hydroxy-4-bromo-2,5-dimethoxyphenylamine or beta-hydroxy 2C-B), is a psychedelic phenethylamine of the 2C family. It is the beta (β) hydroxy structural analogue of 2C-B and is considered a novel psychoactive substance.
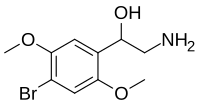 Skeletal formula of βOH-2C-B | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C10H14BrNO3 |
| Molar mass | 276.127 g/mol (freebase) 310.572 g/mol (HCl salt) g·mol−1 |
| 3D model (JSmol) | |
| |
| |
History
editβOH-2C-B is a relatively obscure substance. It appears in a 2004 study on β-oxygenated 5-HT2A serotonin receptor agonists, although it is not mentioned by name.[1] It has occasionally been sold online as research chemical since it began to appear on the grey market in 2020.
Chemistry
editβOH-2C-B is a substituted phenethylamine. It features methoxy substituents at the 2- and 5-position of the ring, as well as a bromine at the 4-position. A hydroxy group is present at the beta (β) position from the functional amine group connected to the alpha (α) carbon, giving rise to its name.
Pharmacology and toxicity
editVery little is known about the pharmacology and toxicity of βOH-2C-B. Its LD50 and toxicity profile have yet to be established.
There are some anecdotal reports of users experiencing headaches or migraines as the drug wears off, which could indicate a toxicity of some kind.[2][unreliable source?]
Legal status
editβOH-2C-B is a controlled substance in the following countries:
- Germany: βOH-2C-B is controlled under the New Psychoactive Substances Act (NpSG) as of November 26, 2016. Possession is illegal but not penalized.[3]
- Netherlands:: βOH-2C-B is not specifically scheduled in the NL but may be considered a ring-derived analogue of 2-phenethylamine which would make it illegal under updates made to the Dutch Opium Act in February 2024.[4]
- United Kingdom: βOH-2C-B is illegal to produce, supply or import under the Psychoactive Substance Act as of May 26, 2016.[5]
- United States: βOH-2C-B is unscheduled in the U.S., but may be considered an analogue of 2C-B under the Federal Analogue Act, and thus a Schedule I drug.[6]
See also
editReferences
edit- ^ Glennon RA, Bondarev ML, Khorana N, Young R, May JA, Hellberg MR, et al. (November 2004). "Beta-oxygenated analogues of the 5-HT2A serotonin receptor agonist 1-(4-bromo-2,5-dimethoxyphenyl)-2-aminopropane". Journal of Medicinal Chemistry. 47 (24): 6034–6041. doi:10.1021/jm040082s. PMID 15537358.
- ^ "BOH-2C-B (also βOH-2C-B; β-OH-2C-B; β-Hydroxy-2C-B) : Erowid Exp: Main Index". www.erowid.org. Retrieved 2024-02-19.
- ^ "NpSG Neue-psychoaktive-Stoffe-Gesetz" [New Psychoactive Substances Act (NpSG)]. Bundesrecht - tagaktuell konsolidiert - alle Fassungen seit 2006 [Federal law - consolidated daily - all versions since 2006] (in German). Retrieved 2024-02-19.
- ^ Ministerie van Binnenlandse Zaken en Koninkrijksrelaties (Ministry of the Interior and Kingdom Relations). "Opiumwet" [Opium Act]. wetten.overheid.nl (in Dutch). Retrieved 2024-02-19.
- ^ "Psychoactive Substances Act 2016". U.K. Home Office. 28 January 2016. Retrieved 8 September 2016.
- ^ "21 U.S. Code § 813 - Treatment of controlled substance analogues". LII / Legal Information Institute. Retrieved 2024-02-19.