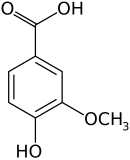
Vanillic acid (4-hydroxy-3-methoxybenzoic acid) is a dihydroxybenzoic acid derivative used as a flavoring agent. It is an oxidized form of vanillin. It is also an intermediate in the production of vanillin from ferulic acid.[2][3]
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
4-Hydroxy-3-methoxybenzoic acid | |||
| Other names
4-Hydroxy-m-anisic acid, Vanillate
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.061 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C8H8O4 | |||
| Molar mass | 168.148 g·mol−1 | ||
| Appearance | White to light yellow powder or crystals | ||
| Melting point | 210 to 213 °C (410 to 415 °F; 483 to 486 K) | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Related compounds
|
Vanillin, vanillyl alcohol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Occurrence in nature
editThe highest amount of vanillic acid in plants known so far is found in the root of Angelica sinensis,[4] an herb indigenous to China, which is used in traditional Chinese medicine.
Occurrences in food
editAçaí oil, obtained from the fruit of the açaí palm (Euterpe oleracea), is rich in vanillic acid (1616±94 mg/kg).[5] It is one of the main natural phenols in argan oil.[citation needed] It is also found in wine and vinegar.[6]
Metabolism
editVanillic acid is one of the main catechins metabolites found in humans after consumption of green tea infusions.[7]
Synthesis
editVanillic acid can be obtained from the oxidation of vanillin by various oxidizing agents. With Pd/C, NaBH4, and KOH as the oxidizing agent, the conversion was reported to occur in ~89% yield.[8]
References
edit- ^ "Vanillic acid (4-hydroxy-3-methoxybenzoic acid)". chemicalland21.com. Retrieved 2009-01-28.
- ^ Lesage-Meessen L, Delattre M, Haon M, Thibault JF, Ceccaldi BC, Brunerie P, Asther M (October 1996). "A two-step bioconversion process for vanillin production from ferulic acid combining Aspergillus niger and Pycnoporus cinnabarinus". J. Biotechnol. 50 (2–3): 107–113. doi:10.1016/0168-1656(96)01552-0. PMID 8987621.
- ^ Civolani C, Barghini P, Roncetti AR, Ruzzi M, Schiesser A (June 2000). "Bioconversion of ferulic acid into vanillic acid by means of a vanillate-negative mutant of Pseudomonas fluorescens strain BF13". Appl. Environ. Microbiol. 66 (6): 2311–2317. Bibcode:2000ApEnM..66.2311C. doi:10.1128/AEM.66.6.2311-2317.2000. PMC 110519. PMID 10831404.
- ^ Duke, JA (1992). Handbook of phytochemical constituents of GRAS herbs and other economic plants. CRC Press, 999 edition. ISBN 978-0-8493-3865-6. Archived from the original on 2015-09-23. Retrieved 2012-01-07.
- ^ Pacheco-Palencia LA, Mertens-Talcott S, Talcott ST (Jun 2008). "Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Açaí (Euterpe oleracea Mart.)". J Agric Food Chem. 56 (12): 4631–4636. doi:10.1021/jf800161u. PMID 18522407.
- ^ Gálvez MC, Barroso CG, Pérez-Bustamante JA (1994). "Analysis of polyphenolic compounds of different vinegar samples". Zeitschrift für Lebensmittel-Untersuchung und -Forschung. 199: 29–31. doi:10.1007/BF01192948. S2CID 91784893.
- ^ Pietta PG, Simonetti P, Gardana C, Brusamolino A, Morazzoni P, Bombardelli E (1998). "Catechin metabolites after intake of green tea infusions". BioFactors. 8 (1–2): 111–8. doi:10.1002/biof.5520080119. PMID 9699018. S2CID 37684286.
- ^ Lim M, Yoon CM, An G, Rhee H (2007). "Environmentally benign oxidation reaction of aldehydes to their corresponding carboxylic acids using Pd/C with NaBH4 and KOH". Tetrahedron Lett. 48 (22): 3835–3839. doi:10.1016/j.tetlet.2007.03.151.