Tungsten(II) iodide is an iodide of tungsten, with the chemical formula [W6I8]I4, or abbreviated as WI2.
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| I2W | |
| Molar mass | 437.65 g·mol−1 |
| Appearance | dark brown solid[1] |
| Density | 6.79 g·cm−3[2] |
| Melting point | 800 °C (decomposes)[2] |
| insoluble[2] | |
| Related compounds | |
Other anions
|
tungsten(II) chloride tungsten(II) bromide |
Other cations
|
chromium(II) iodide molybdenum(II) iodide |
Related compounds
|
tungsten(III) iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation
editTungsten diiodide can obtained from the decomposition from tungsten(III) iodide:[1]
- 6 WI3 → [W6I8]I4 + 3 I2
It can also be formed by the displacement reaction of tungsten(II) chloride and iodine:[1]
- [W6Cl8]Cl4 + 12 I → [W6I8]I4 + 12 Cl
It can also be formed by the direct reaction of tungsten and iodine, which is a reversible reaction. This reaction can be used in halogen lamps.[3]
- W + I2 ⇌ WI2
Tungsten(II) iodide can also be obtained by reacting tungsten hexacarbonyl with iodine.[4]
Properties
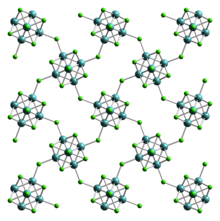
editTungsten(II) iodide is a dark brown-colored solid that is stable in air and moisture. Its structure is the same as tungsten(II) chloride, crystallising orthorhombic crystal system, with space group Bbem (No. 64), and lattice parameters a = 1258 pm, b = 1259 pm, c = 1584 pm.[1]
References
edit- ^ a b c d Handbuch der präparativen anorganischen Chemie. 3 (3., umgearb. Aufl ed.). Stuttgart: Enke. 1981. ISBN 978-3-432-87823-2.
- ^ a b c Haynes, William M.; Lide, David R.; Bruno, Thomas J. (2012). CRC handbook of chemistry and physics: a ready reference book of chemical and physical data (93rd ed.). Boca Raton: CRC. ISBN 978-1-4398-8049-4.
- ^ Latscha, Hans Peter; Mutz, Martin (2011). Chemie der Elemente. Chemie-Basiswissen. Berlin Heidelberg: Springer. ISBN 978-3-642-16915-1.
- ^ Johnson, Brian Frederick Gilbert (1972). Inorganic chemistry of the transition elements. A specialist periodical report. London: Chemical society. ISBN 978-0-85186-500-3.