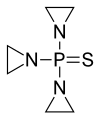
Thiotepa (INN[8]), sold under the brand name Tepadina among others, is an anti-cancer medication.[5][7][9]
| |||
| Clinical data | |||
|---|---|---|---|
| Trade names | Tepadina | ||
| AHFS/Drugs.com | Monograph | ||
| MedlinePlus | a682821 | ||
| License data | |||
| Pregnancy category |
| ||
| Routes of administration | Intravenous, intracavitary, intravesical | ||
| ATC code | |||
| Legal status | |||
| Legal status | |||
| Pharmacokinetic data | |||
| Metabolism | Liver (CYP2B6, CYP3A) | ||
| Elimination half-life | 1.5–4.1 hours | ||
| Excretion | Kidney 6 hours for thiotepa 8 hours for TEPA | ||
| Identifiers | |||
| |||
| CAS Number | |||
| PubChem CID | |||
| IUPHAR/BPS | |||
| DrugBank | |||
| ChemSpider | |||
| UNII | |||
| KEGG | |||
| ChEMBL | |||
| CompTox Dashboard (EPA) | |||
| ECHA InfoCard | 100.000.124 | ||
| Chemical and physical data | |||
| Formula | C6H12N3PS | ||
| Molar mass | 189.22 g·mol−1 | ||
| 3D model (JSmol) | |||
| |||
| |||
| | |||
Thiotepa is an organophosphorus compound with the formula (C2H4N)3PS.[10]
Medical uses
editThiotepa is indicated for use in combination with other chemotherapy agents to treat cancer.[5][7][9] This can be with or without total body irradiation (TBI), as a conditioning treatment prior to allogeneic or autologous hematopoietic progenitor cell transplantation (HPCT) in hematological diseases in adults and children.[7][9] These diseases include Hodgkin's disease and leukaemia.[9] Thiotepa is also used with high-dose chemotherapy with HPCT support to treat certain solid tumors in adult and children.[7][9]
Thiotepa is used in the palliation of many neoplastic diseases. The best results are found in the treatment of adenocarcinoma of the breast, adenocarcinoma of the ovary, papillary thyroid cancer and bladder cancer. Thiotepa is used to control intracavitary effusions caused by serosal neoplastic deposits.[9]
Intravesical use
editThiotepa is used as intravesical chemotherapy in bladder cancer.[11]
Side effects
editThe main side effect of thiotepa is bone marrow suppression resulting in leukopenia, thrombocytopenia and anemia.[12]
History
editThiotepa was developed by the American Cyanamid company in the early 1950s and reported to media outlets in 1953.[13] In 1959, thiotepa was registered with the US Food and Drug Administration (FDA) as a drug therapy for several solid cancers.[14]
In January 2007, the European Medicines Agency (EMA) designated thiotepa as an orphan drug. In April 2007, the United States FDA designated thiotepa as a conditioning treatment for use prior to hematopoietic stem cell transplantation.[15]
In June 2024, the FDA approved a ready-to-dilute liquid formulation of thiotepa to treat breast and ovarian cancer.[16]
References
edit- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Tepadina (Link Medical Products Pty Ltd T/A Link Pharmaceuticals)". Therapeutic Goods Administration (TGA). 28 September 2022. Archived from the original on 18 March 2023. Retrieved 29 April 2023.
- ^ "Cancer therapies". Health Canada. 8 May 2018. Retrieved 13 April 2024.
- ^ "Thiotepa 100 mg powder for concentrate for solution for infusion". (emc). 27 October 2022. Retrieved 14 August 2024.
- ^ a b c "Tepadina- thiotepa injection, powder, for solution". DailyMed. Archived from the original on 12 August 2021. Retrieved 11 August 2021.
- ^ "Highlights of prescribing information" (PDF). www.accessdata.fda.gov.
- ^ a b c d e "Tepadina EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 6 March 2021. Retrieved 30 April 2021.
- ^ "International Non-Proprietary Names for Pharmaceutical Preparations. Recommended International Non-Proprietary Names (Rec. I.N.N.): List 4" (PDF). World Health Organization. March 1962. p. 111. Archived from the original (PDF) on 18 May 2016. Retrieved 27 November 2016.
- ^ a b c d e f "Urgent, Thiotepa update" (PDF). U.S. Food and Drug Administration (FDA). 5 April 2011. Archived (PDF) from the original on 9 November 2011. Retrieved 25 November 2011.
- ^ Maanen MJ, Smeets CJ, Beijnen JH (August 2000). "Chemistry, pharmacology and pharmacokinetics of N,N',N" -triethylenethiophosphoramide (ThioTEPA)". Cancer Treatment Reviews. 26 (4): 257–68. doi:10.1053/ctrv.2000.0170. PMID 10913381.
- ^ Droller M (2004). Urothelial Tumors. PMPH-USA. p. 207. ISBN 978-1-55009-173-1. Archived from the original on 11 January 2023. Retrieved 16 September 2017.
- ^ Agnelli G, de Cunto M, Gresele P, del Favero A (June 1982). "Early onset life-threatening myelosuppression after low dose of intravesical thiotepa". Postgraduate Medical Journal. 58 (680): 380–1. doi:10.1136/pgmj.58.680.380. PMC 2426344. PMID 6812036.
- ^ Sykes MP, Karnofsky DA, Philips FS, Burchenal JH (1953). "Clinical studies on triethylenephosphoramide and diethylenephosphoramide, compounds with nitrogen-mustard-like activity". Cancer. 6 (1): 142–148. doi:10.1002/1097-0142(195301)6:1<142::AID-CNCR2820060114>3.0.CO;2-W.
- ^ Kim KM, Roh JK, Wee H, Kim C (2016). Cancer Drug Discovery: Science and History. Springer. p. 82. ISBN 978-94-024-0844-7.
- ^ "EMA Grants Adienne Marketing Rights for Tepadina". Drug Discovery & Development. 19 March 2010. Retrieved 25 November 2011.
- ^ "Shorla Oncology Announces FDA Approval for Tepylute, A Novel Formulation to Treat Breast and Ovarian Cancer" (Press release). Shorla Oncology. 28 June 2024 – via Business Wire.