RNA-dependent RNA polymerase (RdRp) or RNA replicase is an enzyme that catalyzes the replication of RNA from an RNA template. Specifically, it catalyzes synthesis of the RNA strand complementary to a given RNA template. This is in contrast to typical DNA-dependent RNA polymerases, which all organisms use to catalyze the transcription of RNA from a DNA template.
| RNA-dependent RNA polymerase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
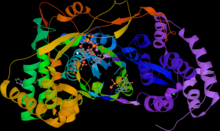 Stalled HCV RNA replicase (NS5B), in complex with sofosbuvir (PDB 4WTG). | |||||||||
| Identifiers | |||||||||
| EC no. | 2.7.7.48 | ||||||||
| CAS no. | 9026-28-2 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
RdRp is an essential protein encoded in the genomes of most RNA-containing viruses that lack a DNA stage,[1][2] including SARS-CoV-2. Some eukaryotes also contain RdRps, which are involved in RNA interference and differ structurally from viral RdRps.
History
editViral RdRps were discovered in the early 1960s from studies on mengovirus and polio virus when it was observed that these viruses were not sensitive to actinomycin D, a drug that inhibits cellular DNA-directed RNA synthesis. This lack of sensitivity suggested the action of a virus-specific enzyme that could copy RNA from an RNA template.[3]
Distribution
editRdRps are highly conserved in viruses and are related to telomerase, though the reason for this was an ongoing question as of 2009.[4] The similarity led to speculation that viral RdRps are ancestral to human telomerase.[5]
The most famous example of RdRp is in the polio virus. The viral genome is composed of RNA, which enters the cell through receptor-mediated endocytosis. From there, the RNA acts as a template for complementary RNA synthesis. The complementary strand acts as a template for the production of new viral genomes that are packaged and released from the cell ready to infect more host cells. The advantage of this method of replication is that no DNA stage complicates replication. The disadvantage is that no 'back-up' DNA copy is available.[6]
Many RdRps associate tightly with membranes making them difficult to study. The best-known RdRps are polioviral 3Dpol, vesicular stomatitis virus L,[7] and hepatitis C virus NS5B protein.
Many eukaryotes have RdRps that are involved in RNA interference: these amplify microRNAs and small temporal RNAs and produce double-stranded RNA using small interfering RNAs as primers.[8] These RdRps are used in the defense mechanisms and can be appropriated by RNA viruses.[9] Their evolutionary history predates the divergence of major eukaryotic groups.[10]
Replication
editRdRp differs from DNA dependent RNA polymerase as it catalyzes RNA synthesis of strands complementary to a given RNA template. The RNA replication process is a four-step mechanism:
- Nucleoside triphosphate (NTP) binding – initially, the RdRp presents with a vacant active site in which an NTP binds, complementary to the corresponding nucleotide on the template strand. Correct NTP binding causes the RdRp to undergo a conformational change.[11]
- Active site closure – the conformational change, initiated by the correct NTP binding, results in the restriction of active site access and produces a catalytically competent state.[11]
- Phosphodiester bond formation – two Mg2+ ions are present in the catalytically active state and arrange themselves around the newly synthesized RNA chain such that the substrate NTP undergoes a phosphatidyl transfer and forms a phosphodiester bond with the new chain.[12] Without the use of these Mg2+ ions, the active site is no longer catalytically stable and the RdRp complex changes to an open conformation.[12]
- Translocation – once the active site is open, the RNA template strand moves by one position through the RdRp protein complex and continues chain elongation by binding a new NTP, unless otherwise specified by the template.[11]
RNA synthesis can be performed by a primer-independent (de novo) or a primer-dependent mechanism that utilizes a viral protein genome-linked (VPg) primer.[13] The de novo initiation consists in the addition of a NTP to the 3'-OH of the first initiating NTP.[13] During the following elongation phase, this nucleotidyl transfer reaction is repeated with subsequent NTPs to generate the complementary RNA product. Termination of the nascent RNA chain produced by RdRp is not completely known, however, RdRp termination is sequence-independent.[14]
One major drawback of RNA-dependent RNA polymerase replication is the transcription error rate.[13] RdRps lack fidelity on the order of 104 nucleotides, which is thought to be a direct result of inadequate proofreading.[13] This variation rate is favored in viral genomes as it allows for the pathogen to overcome host defenses trying to avoid infection, allowing for evolutionary growth.[15]
Structure
editViral/prokaryotic RdRp, along with many single-subunit DdRp, employ a fold whose organization has been linked to the shape of a right hand with three subdomains termed fingers, palm, and thumb.[16] Only the palm subdomain, composed of a four-stranded antiparallel beta sheet with two alpha helices, is well conserved. In RdRp, the palm subdomain comprises three well-conserved motifs (A, B, and C). Motif A (D-x(4,5)-D) and motif C (GDD) are spatially juxtaposed; the aspartic acid residues of these motifs are implied in the binding of Mg2+ and/or Mn2+. The asparagine residue of motif B is involved in selection of ribonucleoside triphosphates over dNTPs and, thus, determines whether RNA rather than DNA is synthesized.[17] The domain organization[18] and the 3D structure of the catalytic centre of a wide range of RdRps, even those with a low overall sequence homology, are conserved. The catalytic center is formed by several motifs containing conserved amino acid residues.[citation needed]
Eukaryotic RNA interference requires a cellular RdRp (c RdRp). Unlike the "hand" polymerases, they resemble simplified multi-subunit DdRPs, specifically in the catalytic β/β' subunits, in that they use two sets of double-psi β-barrels in the active site. QDE1 (Q9Y7G6) in Neurospora crassa, which has both barrels in the same chain,[19] is an example of such a c RdRp enzyme.[20] Bacteriophage homologs of c RdRp, including the similarly single-chain DdRp yonO (O31945), appear to be closer to c RdRps than DdRPs are.[8][21]
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Viruses
editFour superfamilies of viruses cover all RNA-containing viruses with no DNA stage:
- Viruses containing positive-strand RNA or double-strand RNA, except retroviruses and Birnaviridae
- All positive-strand RNA eukaryotic viruses with no DNA stage, such as Coronaviridae
- All RNA-containing bacteriophages; the two families of RNA-containing bacteriophages are Fiersviridae (positive ssRNA phages) and Cystoviridae (dsRNA phages)
- dsRNA virus family Reoviridae, Totiviridae, Hypoviridae, Partitiviridae
- Mononegavirales (negative-strand RNA viruses with non-segmented genomes; InterPro: IPR016269)
- Negative-strand RNA viruses with segmented genomes (InterPro: IPR007099), such as orthomyxoviruses and bunyaviruses
- dsRNA virus family Birnaviridae (InterPro: IPR007100)
Flaviviruses produce a polyprotein from the ssRNA genome. The polyprotein is cleaved to a number of products, one of which is NS5, an RdRp. It possesses short regions and motifs homologous to other RdRps.[22]
RNA replicase found in positive-strand ssRNA viruses are related to each other, forming three large superfamilies.[23] Birnaviral RNA replicase is unique in that it lacks motif C (GDD) in the palm.[24] Mononegaviral RdRp (PDB 5A22) has been automatically classified as similar to (+)−ssRNA RdRps, specifically one from Pestivirus and one from Leviviridae.[25] Bunyaviral RdRp monomer (PDB 5AMQ) resembles the heterotrimeric complex of Orthomyxoviral (Influenza; PDB 4WSB) RdRp.[26]
Since it is a protein universal to RNA-containing viruses, RdRp is a useful marker for understanding their evolution.[27][28]
Recombination
editWhen replicating its (+)ssRNA genome, the poliovirus RdRp is able to carry out recombination. Recombination appears to occur by a copy choice mechanism in which the RdRp switches (+)ssRNA templates during negative strand synthesis.[29] Recombination frequency is determined in part by the fidelity of RdRp replication.[30] RdRp variants with high replication fidelity show reduced recombination, and low fidelity RdRps exhibit increased recombination.[30] Recombination by RdRp strand switching occurs frequently during replication in the (+)ssRNA plant carmoviruses and tombusviruses.[31]
Intragenic complementation
editSendai virus (family Paramyxoviridae) has a linear, single-stranded, negative-sense, nonsegmented RNA genome. The viral RdRp consists of two virus-encoded subunits, a smaller one P and a larger one L. Testing different inactive RdRp mutants with defects throughout the length of the L subunit in pairwise combinations, restoration of viral RNA synthesis was observed in some combinations.[32] This positive L–L interaction is referred to as intragenic complementation and indicates that the L protein is an oligomer in the viral RNA polymerase complex.[citation needed]
Drug therapies
edit- RdRps can be used as drug targets for viral pathogens as their function is not necessary for eukaryotic survival. By inhibiting RdRp function, new RNAs cannot be replicated from an RNA template strand, however, DNA-dependent RNA polymerase remains functional.
- Some antiviral drugs against Hepatitis C and COVID-19 specifically target RdRp. These include Sofosbuvir and Ribavirin against Hepatitis C[33] and remdesivir, an FDA approved drug against COVID-19
- GS-441524 triphosphate is a substrate for RdRp, but not mammalian polymerases. It results in premature chain termination and inhibition of viral replication. GS-441524 triphosphate is the biologically active form of remdesivir. Remdesivir is classified as a nucleotide analog that inhibits RdRp function by covalently binding to and interrupting termination of the nascent RNA through early or delayed termination or preventing further elongation of the RNA polynucleotide.[34][35] This early termination leads to nonfunctional RNA that gets degraded through normal cellular processes.
RNA interference
editThe use of RdRp plays a major role in RNA interference in eukaryotes, a process used to silence gene expression via small interfering RNAs (siRNAs) binding to mRNA rendering them inactive.[36] Eukaryotic RdRp becomes active in the presence of dsRNA, and is less widely distributed than other RNAi components as it lost in some animals, though still found in C. elegans, P. tetraurelia,[37] and plants.[38] This presence of dsRNA triggers the activation of RdRp and RNAi processes by priming the initiation of RNA transcription through the introduction of siRNAs.[37] In C. elegans, siRNAs are integrated into the RNA-induced silencing complex, RISC, which works alongside mRNAs targeted for interference to recruit more RdRps to synthesize more secondary siRNAs and repress gene expression.[39]
See also
editNotes
editReferences
edit- ^ Koonin EV, Gorbalenya AE, Chumakov KM (July 1989). "Tentative identification of RNA-dependent RNA polymerases of dsRNA viruses and their relationship to positive strand RNA viral polymerases". FEBS Letters. 252 (1–2): 42–46. Bibcode:1989FEBSL.252...42K. doi:10.1016/0014-5793(89)80886-5. PMID 2759231. S2CID 36482110.
- ^ Zanotto PM, Gibbs MJ, Gould EA, Holmes EC (September 1996). "A reevaluation of the higher taxonomy of viruses based on RNA polymerases". Journal of Virology. 70 (9): 6083–6096. doi:10.1128/JVI.70.9.6083-6096.1996. PMC 190630. PMID 8709232.
- ^ Baltimore D, Franklin RM (October 1963). "A New Ribonucleic Acid Polymerase Appearing after Mengovirus Infection of L-Cells". The Journal of Biological Chemistry. 238 (10): 3395–3400. doi:10.1016/S0021-9258(18)48679-6. PMID 14085393.
- ^ Suttle CA (September 2005). "Viruses in the sea". Nature. 437 (7057): 356–361. Bibcode:2005Natur.437..356S. doi:10.1038/nature04160. PMID 16163346. S2CID 4370363.
- ^ Weiner AM (January 1988). "Eukaryotic nuclear telomeres: molecular fossils of the RNP world?". Cell. 52 (2): 155–158. doi:10.1016/0092-8674(88)90501-6. PMID 2449282. S2CID 11491076.
- ^ Dawkins R (1996). The Blind Watchmaker (PDF) (3d ed.). London: W.W. Norton&Company. p. 129. ISBN 978-0-393-35309-9.
- ^ Timm C, Gupta A, Yin J (August 2015). "Robust kinetics of an RNA virus: Transcription rates are set by genome levels". Biotechnology and Bioengineering. 112 (8): 1655–1662. doi:10.1002/bit.25578. PMC 5653219. PMID 25726926.
- ^ a b Iyer LM, Koonin EV, Aravind L (January 2003). "Evolutionary connection between the catalytic subunits of DNA-dependent RNA polymerases and eukaryotic RNA-dependent RNA polymerases and the origin of RNA polymerases". BMC Structural Biology. 3: 1. doi:10.1186/1472-6807-3-1. PMC 151600. PMID 12553882.
- ^ Tan FL, Yin JQ (December 2004). "RNAi, a new therapeutic strategy against viral infection". Cell Research. 14 (6): 460–466. doi:10.1038/sj.cr.7290248. PMC 7092015. PMID 15625012.
- ^ Zong J, Yao X, Yin J, Zhang D, Ma H (November 2009). "Evolution of the RNA-dependent RNA polymerase (RdRP) genes: duplications and possible losses before and after the divergence of major eukaryotic groups". Gene. 447 (1): 29–39. doi:10.1016/j.gene.2009.07.004. PMID 19616606.
- ^ a b c Wu J, Gong P (January 2018). "Visualizing the Nucleotide Addition Cycle of Viral RNA-Dependent RNA Polymerase". Viruses. 10 (1): 24. doi:10.3390/v10010024. PMC 5795437. PMID 29300357.
- ^ a b Shu B, Gong P (July 2016). "Structural basis of viral RNA-dependent RNA polymerase catalysis and translocation". Proceedings of the National Academy of Sciences of the United States of America. 113 (28): E4005–E4014. Bibcode:2016PNAS..113E4005S. doi:10.1073/pnas.1602591113. PMC 4948327. PMID 27339134.
- ^ a b c d Venkataraman S, Prasad BV, Selvarajan R (February 2018). "RNA Dependent RNA Polymerases: Insights from Structure, Function and Evolution". Viruses. 10 (2): 76. doi:10.3390/v10020076. PMC 5850383. PMID 29439438.
- ^ Adkins S, Stawicki SS, Faurote G, Siegel RW, Kao CC (April 1998). "Mechanistic analysis of RNA synthesis by RNA-dependent RNA polymerase from two promoters reveals similarities to DNA-dependent RNA polymerase". RNA. 4 (4): 455–470. PMC 1369631. PMID 9630251.
- ^ Fitzsimmons WJ, Woods RJ, McCrone JT, Woodman A, Arnold JJ, Yennawar M, et al. (June 2018). "A speed-fidelity trade-off determines the mutation rate and virulence of an RNA virus". PLOS Biology. 16 (6): e2006459. doi:10.1371/journal.pbio.2006459. PMC 6040757. PMID 29953453.
- ^ Hansen JL, Long AM, Schultz SC (August 1997). "Structure of the RNA-dependent RNA polymerase of poliovirus". Structure. 5 (8): 1109–1122. doi:10.1016/S0969-2126(97)00261-X. PMID 9309225.
- ^ Gohara DW, Crotty S, Arnold JJ, Yoder JD, Andino R, Cameron CE (August 2000). "Poliovirus RNA-dependent RNA polymerase (3Dpol): structural, biochemical, and biological analysis of conserved structural motifs A and B". The Journal of Biological Chemistry. 275 (33): 25523–25532. doi:10.1074/jbc.M002671200. PMID 10827187.
- ^ O'Reilly EK, Kao CC (December 1998). "Analysis of RNA-dependent RNA polymerase structure and function as guided by known polymerase structures and computer predictions of secondary structure". Virology. 252 (2): 287–303. doi:10.1006/viro.1998.9463. PMID 9878607.
- ^ Sauguet L (September 2019). "The Extended "Two-Barrel" Polymerases Superfamily: Structure, Function and Evolution". Journal of Molecular Biology. 431 (20): 4167–4183. doi:10.1016/j.jmb.2019.05.017. PMID 31103775.
- ^ Werner F, Grohmann D (February 2011). "Evolution of multisubunit RNA polymerases in the three domains of life". Nature Reviews. Microbiology. 9 (2): 85–98. doi:10.1038/nrmicro2507. PMID 21233849. S2CID 30004345.
- ^ Forrest D, James K, Yuzenkova Y, Zenkin N (June 2017). "Single-peptide DNA-dependent RNA polymerase homologous to multi-subunit RNA polymerase". Nature Communications. 8: 15774. Bibcode:2017NatCo...815774F. doi:10.1038/ncomms15774. PMC 5467207. PMID 28585540.
- ^ Tan BH, Fu J, Sugrue RJ, Yap EH, Chan YC, Tan YH (February 1996). "Recombinant dengue type 1 virus NS5 protein expressed in Escherichia coli exhibits RNA-dependent RNA polymerase activity". Virology. 216 (2): 317–325. doi:10.1006/viro.1996.0067. PMID 8607261.
- ^ Koonin EV (September 1991). "The phylogeny of RNA-dependent RNA polymerases of positive-strand RNA viruses". The Journal of General Virology. 72 ( Pt 9) (9): 2197–2206. doi:10.1099/0022-1317-72-9-2197. PMID 1895057.
- ^ Shwed PS, Dobos P, Cameron LA, Vakharia VN, Duncan R (May 2002). "Birnavirus VP1 proteins form a distinct subgroup of RNA-dependent RNA polymerases lacking a GDD motif". Virology. 296 (2): 241–250. doi:10.1006/viro.2001.1334. PMID 12069523.
- ^ Structural Similarities for the Entities in PDB 5A22 Archived 2019-04-03 at the Wayback Machine.
- ^ Gerlach P, Malet H, Cusack S, Reguera J (June 2015). "Structural Insights into Bunyavirus Replication and Its Regulation by the vRNA Promoter". Cell. 161 (6): 1267–1279. doi:10.1016/j.cell.2015.05.006. PMC 4459711. PMID 26004069.
- ^ Wolf YI, Kazlauskas D, Iranzo J, Lucía-Sanz A, Kuhn JH, Krupovic M, et al. (November 2018). "Origins and Evolution of the Global RNA Virome". mBio. 9 (6). doi:10.1128/mBio.02329-18. PMC 6282212. PMID 30482837.
- ^ Černý J, Černá Bolfíková B, Valdés JJ, Grubhoffer L, Růžek D (2014). "Evolution of tertiary structure of viral RNA dependent polymerases". PLOS ONE. 9 (5): e96070. Bibcode:2014PLoSO...996070C. doi:10.1371/journal.pone.0096070. PMC 4015915. PMID 24816789.
- ^ Kirkegaard K, Baltimore D (November 1986). "The mechanism of RNA recombination in poliovirus". Cell. 47 (3): 433–443. doi:10.1016/0092-8674(86)90600-8. PMC 7133339. PMID 3021340.
- ^ a b Woodman A, Arnold JJ, Cameron CE, Evans DJ (August 2016). "Biochemical and genetic analysis of the role of the viral polymerase in enterovirus recombination". Nucleic Acids Research. 44 (14): 6883–6895. doi:10.1093/nar/gkw567. PMC 5001610. PMID 27317698.
- ^ Cheng CP, Nagy PD (November 2003). "Mechanism of RNA recombination in carmo- and tombusviruses: evidence for template switching by the RNA-dependent RNA polymerase in vitro". Journal of Virology. 77 (22): 12033–12047. doi:10.1128/jvi.77.22.12033-12047.2003. PMC 254248. PMID 14581540.
- ^ Smallwood S, Cevik B, Moyer SA (December 2002). "Intragenic complementation and oligomerization of the L subunit of the sendai virus RNA polymerase". Virology. 304 (2): 235–245. doi:10.1006/viro.2002.1720. PMID 12504565.
- ^ Waheed Y, Bhatti A, Ashraf M (March 2013). "RNA dependent RNA polymerase of HCV: a potential target for the development of antiviral drugs". Infection, Genetics and Evolution. 14: 247–257. Bibcode:2013InfGE..14..247W. doi:10.1016/j.meegid.2012.12.004. PMID 23291407.
- ^ Yin W, Mao C, Luan X, Shen DD, Shen Q, Su H, et al. (June 2020). "Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir". Science. 368 (6498): 1499–1504. Bibcode:2020Sci...368.1499Y. doi:10.1126/science.abc1560. PMC 7199908. PMID 32358203.
- ^ Malin JJ, Suárez I, Priesner V, Fätkenheuer G, Rybniker J (December 2020). "Remdesivir against COVID-19 and Other Viral Diseases". Clinical Microbiology Reviews. 34 (1). doi:10.1128/CMR.00162-20. PMC 7566896. PMID 33055231.
- ^ Simaan JA, Aviado DM (November 1975). "Hemodynamic effects of aerosol propellants. II. Pulmonary circulation in the dog". Toxicology. 5 (2): 139–146. Bibcode:1975Toxgy...5..139S. doi:10.1016/0300-483x(75)90110-9. PMID 1873.
- ^ a b Marker S, Le Mouël A, Meyer E, Simon M (July 2010). "Distinct RNA-dependent RNA polymerases are required for RNAi triggered by double-stranded RNA versus truncated transgenes in Paramecium tetraurelia". Nucleic Acids Research. 38 (12): 4092–4107. doi:10.1093/nar/gkq131. PMC 2896523. PMID 20200046.
- ^ Willmann MR, Endres MW, Cook RT, Gregory BD (July 2011). "The Functions of RNA-Dependent RNA Polymerases in Arabidopsis". The Arabidopsis Book. 9: e0146. doi:10.1199/tab.0146. PMC 3268507. PMID 22303271.
- ^ Zhang C, Ruvkun G (August 2012). "New insights into siRNA amplification and RNAi". RNA Biology. 9 (8): 1045–1049. doi:10.4161/rna.21246. PMC 3551858. PMID 22858672.
External links
edit- RNA+Replicase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- EC 2.7.7.48