Lisinopril/hydrochlorothiazide, sold under the brand name Zestoretic among others, is a fixed-dose combination medication used for the treatment of high blood pressure (hypertension).[2] It contains lisinopril, an ACE inhibitor, and hydrochlorothiazide, a diuretic.[2][3] Typically, it becomes an option once a person is doing well on the individual components.[4] It is taken by mouth.[3]
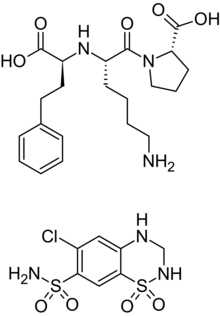 Lisinopril (top) and hydrochlorothiazide (bottom) | |
| Combination of | |
|---|---|
| Lisinopril | ACE inhibitor |
| Hydrochlorothiazide | Thiazide diuretic |
| Clinical data | |
| Trade names | Zestoretic, Prinzide, others |
| Other names | lisinopril/hctz |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a601070 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| KEGG | |
| (verify) | |
Common side effects include dizziness, headache, cough, and feeling tired.[2] Severe side effects may include angioedema and low blood pressure.[2] Use during pregnancy may harm the baby.[2]
The combination was approved for medical use in the United States in 1989.[3] It is on the World Health Organization's List of Essential Medicines.[5] It is available as a generic medication.[4] In 2022, the combination was the 53rd most commonly prescribed medication in the United States, with more than 12 million prescriptions.[6][7]
Medical uses
editLisinopril/hydrochlorothiazide is indicated for the treatment of hypertension, to lower blood pressure.[2]
Adverse effects
editThe US Food and Drug Administration prescription label for the combination contains a boxed warning about harm to the baby.[2]
References
edit- ^ "Product monograph brand safety updates". Health Canada. February 2024. Retrieved 24 March 2024.
- ^ a b c d e f g h "Zestoretic- lisinopril and hydrochlorothiazide tablet". DailyMed. Retrieved 9 February 2022.
- ^ a b c "Hydrochlorothiazide and lisinopril Uses, Side Effects & Warnings". Drugs.com. Cerner Multum. Retrieved 26 December 2018.
- ^ a b British national formulary : BNF 74 (74 ed.). British Medical Association. 2017. p. 166. ISBN 978-0857112989.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Hydrochlorothiazide; Lisinopril Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.