Oxygen therapy, also referred to as supplemental oxygen, is the use of oxygen as medical treatment.[1] Supplemental oxygen can also refer to the use of oxygen enriched air at altitude. Acute indications for therapy include hypoxemia (low blood oxygen levels), carbon monoxide toxicity and cluster headache. It may also be prophylactically given to maintain blood oxygen levels during the induction of anesthesia.[2] Oxygen therapy is often useful in chronic hypoxemia caused by conditions such as severe COPD or cystic fibrosis.[3][1] Oxygen can be delivered via nasal cannula, face mask, or endotracheal intubation at normal atmospheric pressure, or in a hyperbaric chamber.[4][5] It can also be given through bypassing the airway, such as in ECMO therapy.
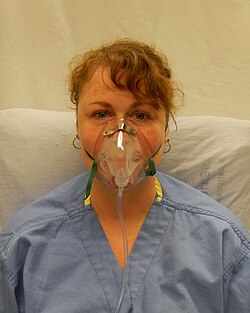 A person wearing a simple face mask | |
| Clinical data | |
|---|---|
| Other names | supplemental oxygen, enriched air |
| AHFS/Drugs.com | FDA Professional Drug Information |
| Routes of administration | inhaled |
| Drug class | medical gas |
| ATC code | |
| Identifiers | |
| CAS Number | |
| ChemSpider |
|
| UNII | |
| Chemical and physical data | |
| Formula | O2 |
Oxygen is required for normal cellular metabolism.[6] However, excessively high concentrations can result in oxygen toxicity, leading to lung damage and respiratory failure.[2][7] Higher oxygen concentrations can also increase the risk of airway fires, particularly while smoking.[1] Oxygen therapy can also dry out the nasal mucosa without humidification.[1] In most conditions, an oxygen saturation of 94–96% is adequate, while in those at risk of carbon dioxide retention, saturations of 88–92% are preferred.[1][8] In cases of carbon monoxide toxicity or cardiac arrest, saturations should be as high as possible.[1][8] While air is typically 21% oxygen by volume, oxygen therapy can increase O2 content of air up to 100%.[7]
The medical use of oxygen first became common around 1917, and is the most common hospital treatment in the developed world.[1][9][10][11] It is currently on the World Health Organization's List of Essential Medicines.[11] Home oxygen can be provided either by oxygen tanks or oxygen concentrator.[1]
Medical uses
editOxygen is widely used by hospitals, EMS, and first-aid providers in a variety of conditions and settings. A few indications frequently requiring high-flow oxygen include resuscitation, major trauma, anaphylaxis, major bleeding, shock, active convulsions, and hypothermia.[12][13]
Acute conditions
editIn context of acute hypoxemia, oxygen therapy should be titrated to a target level based on pulse oximetry (94–96% in most patients, or 88–92% in people with COPD).[12][8] This can be performed by increasing oxygen delivery, described as FIO2(fraction of inspired oxygen). In 2018, the British Medical Journal recommended that oxygen therapy be stopped for saturations greater than 96% and not started for saturations above 90 to 93%.[14] This may be due to an association between excessive oxygenation in the acutely ill and increased mortality.[8] Exceptions to these recommendations include carbon monoxide poisoning, cluster headaches, sickle cell crisis, and pneumothorax.[14]
Oxygen therapy has also been used as emergency treatment for decompression sickness for years.[15] Recompression in a hyperbaric chamber with 100% oxygen is the standard treatment for decompression illness.[15][16][17] The success of recompression therapy is greatest if given within four hours after resurfacing, with earlier treatment associated with a decreased number of recompression treatments required for resolution.[18] It has been suggested in literature that heliox may be a better alternative to oxygen therapy.[19]
In the context of stroke, oxygen therapy may be beneficial as long as hyperoxic environments are avoided.[20]
People receiving outpatient oxygen therapy for hypoxemia following acute illness or hospitalization should be re-assessed by a physician prior to prescription renewal to gauge the necessity of ongoing oxygen therapy.[21] If the initial hypoxemia has resolved, additional treatment may be an unnecessary use of resources.[21]
Chronic conditions
editCommon conditions which may require a baseline of supplementary oxygen include chronic obstructive pulmonary disease (COPD), chronic bronchitis, and emphysema. Patients may also require additional oxygen during acute exacerbations. Oxygen may also be prescribed for breathlessness, end-stage cardiac failure, respiratory failure, advanced cancer, or neurodegenerative disease in spite of relatively normal blood oxygen levels. Physiologically, it may be indicated in people with arterial oxygen partial pressure PaO
2 ≤ 55mmHg (7.3kPa) or arterial oxygen saturation SaO
2 ≤ 88%.[22][23][24]
Careful titration of oxygen therapy should be considered in patients with chronic conditions predisposing them to carbon dioxide retention (e.g., COPD, emphysema). In these instances, oxygen therapy may decrease respiratory drive, leading to accumulation of carbon dioxide (hypercapnia), acidemia, and increased mortality secondary to respiratory failure.[25] Improved outcomes have been observed with titrated oxygen treatment largely due to gradual improvement of the ventilation/perfusion ratio.[26] The risks associated with loss of respiratory drive are far outweighed by the risks of withholding emergency oxygen, so emergency administration of oxygen is never contraindicated. Transfer from the field to definitive care with titrated oxygen typically occurs long before significant reductions to the respiratory drive are observed.[citation needed]
Contraindications
editThere are certain situations in which oxygen therapy has been shown to negatively impact a person's condition.[27]
- Oxygen therapy can exacerbate the effects of paraquat poisoning and should be withheld unless severe respiratory distress or respiratory arrest is present. Paraquat poisoning is rare, with about 200 deaths globally from 1958 to 1978.[28]
- Oxygen therapy is not recommended for people with pulmonary fibrosis or bleomycin-associated lung damage.[29]
- ARDS caused by acid aspiration may be exacerbated with oxygen therapy according to some animal studies.[30][31]
- Hyperoxic environments should be avoided in cases of sepsis.[20]
Pin-indexed Oxygen Regulator for portable D-Cylinder, usually carried in an ambulance's resuscitation kit
This section needs expansion with: where possible, explain why these contraindications exist, that would be also be encyclopedic knowledge. You can help by adding to it. (December 2022) |
Adverse effects
editIn some instances, oxygen delivery can lead to particular complications in population subsets.
- In infants with respiratory failure, administration of high levels of oxygen can sometimes promote overgrowth of new blood vessels in the eye leading to blindness. This phenomenon is known as retinopathy of prematurity (ROP).[citation needed]
- In rare instances, people receiving hyperbaric oxygen therapy have had seizures, which has been previously attributed to oxygen toxicity.[32][33]
- There is some evidence that extended HBOT can accelerate development of cataracts.[citation needed]
Alternative medicine
editSome practitioners of alternative medicine have promoted "oxygen therapy" as a cure for many human ailments including AIDS, Alzheimer's disease and cancer. According to the American Cancer Society, "available scientific evidence does not support claims that putting oxygen-releasing chemicals into a person's body is effective in treating cancer", and some of these treatments can be dangerous.[34]
Physiologic effects
editOxygen supplementation has a variety of physiologic effects on the human body. Whether or not these effects are adverse to a patient is dependent upon clinical context. Cases in which an excess amount of oxygen is available to organs is known as hyperoxia.[35] While the following effects may observed with noninvasive high-dose oxygen therapy (i.e., not ECMO), delivery of oxygen at higher pressures is associated with exacerbation of the following associated effects.[citation needed]
Absorption atelectasis
editIt has been hypothesized that oxygen therapy may promote accelerated development of atelectasis (partial or complete lung collapse), as well as denitrogenation of gas cavities (e.g., pneumothorax, pneumocephalus).[36][37] This concept is based on the idea that oxygen is more quickly absorbed compared to nitrogen within the body, leading oxygen-rich areas that are poorly ventilated to be rapidly absorbed, leading to atelectasis.[36] It is thought that higher fractions of inhaled oxygen (FIO2) are associated with increasing rates of atelectasis in the clinical scenario.[38] In clinically healthy adults, it is believed that absorption atelectasis typically does not have any significant implications when managed properly.[39]
Airway inflammation
editIn regard to the airway, both tracheobronchitis and mucositis have been observed with high levels of oxygen delivery (typically >40% O2).[40] Within the lungs, these elevated concentrations of oxygen have been associated with increased alveolar toxicity (coined the Lorrain-Smith effect).[35] Mucosal damage is observed to increase with elevated atmospheric pressure and oxygen concentrations, which may result in the development of ARDS and possibly death.[41][42]
Central nervous system effects
editDecreased cerebral blood flow and intracranial pressure (ICP) have been reported in hyperoxic conditions, with mixed results regarding impact on cognition.[43][44][45][46] Hyperoxia as also been associated with seizures, cataract formation, and reversible myopia.[47]
Hypercapnea
editAmong CO2 retainers, excess exposure to oxygen in context of the Haldane effect causes decreased binding of deoxyhemoglobin to CO2 in the blood.[48] This unloading of CO2 may contribute to the development of acid-base disorders due to the associated increase in PaCO2 (hypercapnea). Patients with underlying lung disease such as COPD may not be able to adequately clear the additional CO2 produced by this effect, worsening their condition.[49] In addition, oxygen therapy has also been shown to decrease respiratory drive, further contributing to possible hypercapnea.[37]
Immunological effects
editHyperoxic environments have been observed to decrease granulocyte rolling and diapedesis in specific circumstances in humans.[50] In regard to anaerobic infections, cases of necrotizing fasciitis have been observed to require fewer debridement operations and have improvement in regard to mortality in patients treated with hyperbaric oxygen therapy.[51] This may stem from oxygen intolerance of otherwise anaerobic microorganisms.[citation needed]
Oxidative stress
editSustained exposure to oxygen may overwhelm the body's capacity to deal with oxidative stress.[52] Rates of oxidative stress appears to be influenced by both oxygen concentration and length of exposure, with general toxicity observed to occur within hours in certain hyperoxic conditions.[53]
Reduction in erythropoiesis
editHyperoxia is observed to result in a serum reduction in erythropoietin, resulting in reduced stimulus for erythropoiesis.[54] Hyperoxia at normobaric environments does not appear to be able to halt erythropoiesis completely.[54]
Pulmonary vasodilation
editWithin the lungs, hypoxia is observed to be a potent pulmonary vasoconstrictor, due to inhibition of an outward potassium current and activation of inward sodium current leading to pulmonary vascular muscular contraction.[55] However, the effects of hyperoxia do not seem to have a particularly strong vasodilatory effect from the few studies that have been performed on patients with pulmonary hypertension.[56][57] As a result, an effect appears to be present but minor.[56][57]
Systemic vasoconstriction
editIn the systemic vasculature, oxygen serves as a vasoconstrictor, leading to mildly increased blood pressure and decreased cardiac output and heart rate. Hyperbaric conditions do not seem to have a significant impact on these overall physiologic effects.[58][46] Clinically, this may lead to increased left-to-right shunting in certain patient populations, such as those with atrial septal defect. While the mechanism of the vasoconstriction is unknown, one proposed theory is that increased reactive oxygen species from oxygen therapy accelerates the degradation of endothelial nitric oxide, a vasodilator.[59][46] These vasoconstrictive effects are thought to be the underlying mechanism helping to abort cluster headaches.[60]
Dissolved oxygen in hyperoxic conditions may make also a significant contribution to total gas transport.[61]
Storage and sources
editOxygen can be separated by a number of methods (e.g., chemical reaction, fractional distillation) to enable immediate or future use. The main methods utilized for oxygen therapy include:
- Liquid storage – Liquid oxygen is stored in insulated tanks at low temperature and allowed to boil (at a temperature of 90.188 K (−182.96 °C)) during use, releasing gaseous oxygen. This method is widely utilized at hospitals due to high oxygen requirements. See Vacuum Insulated Evaporator for more information on this method of storage.
- Compressed gas storage – Oxygen gas is compressed in a gas cylinder, which provides a convenient storage method (refrigeration not required). Large oxygen cylinders hold a volume of 6,500 litres (230 cu ft) and can last about two days at a flow rate of 2 litres per minute (LPM). A small portable M6 (B) cylinder holds 164 or 170 litres (5.8 or 6.0 cu ft) and weighs about 1.3 to 1.6 kilograms (2.9 to 3.5 lb).[62] These tanks can last 4–6 hours with a conserving regulator,[clarification needed] which adjust flow based on a person's breathing rate. Conserving regulators may not be effective for patients who breathe through their mouth.[clarification needed]
- Instant usage – The use of an electrically powered oxygen concentrator[63] or a chemical reaction based unit[64] can create sufficient oxygen for immediate personal use. These units (especially the electrically powered versions) are widely used for home oxygen therapy as portable personal oxygen. One particular advantage includes continuous supply without need for bulky oxygen cylinders.
Hazards and risk
editHighly concentrated sources of oxygen also increase risk for rapid combustion. Oxygen itself is not flammable, but the addition of concentrated oxygen to a fire greatly increases its intensity, and can aid the combustion of materials that are relatively inert under normal conditions. Fire and explosion hazards exist when concentrated oxidants and fuels are brought together in close proximity, although an ignition event (e.g., heat or spark) is needed to trigger combustion.[65]
Concentrated oxygen will allow combustion to proceed rapidly and energetically.[65] Steel pipes and storage vessels used to store and transmit both gaseous and liquid oxygen will act as a fuel; and therefore the design and manufacture of oxygen systems requires special training to ensure that ignition sources are minimized.[65] Highly concentrated oxygen in a high-pressure environment can spontaneously ignite hydrocarbons such as oil and grease, resulting in a fire or explosion. The heat caused by rapid pressurization serves as the ignition source. For this reason, storage vessels, regulators, piping and any other equipment used with highly concentrated oxygen must be "oxygen-clean" prior to use to ensure the absence of potential fuels. This does not only apply to pure oxygen; any concentration significantly higher than atmospheric (approximately 21%) carries a potential ignition risk.[citation needed]
Some hospitals have instituted "no-smoking" policies which can help keep ignition sources away from medically piped oxygen. These policies do not eliminate the risk of injury among patients with portable oxygen systems, especially among smokers.[66] Other potential sources of ignition include candles, aromatherapy, medical equipment, cooking, and deliberate vandalism.[citation needed]
Delivery
editVarious devices are used for oxygen administration. In most cases, the oxygen will first pass through a pressure regulator, used to control the high pressure of oxygen delivered from a cylinder (or other source) to a lower pressure. This lower pressure is then controlled by a flowmeter (which may be preset or selectable) which controls the flow at a measured rate (e.g., litres per minute [LPM]). The typical flowmeter range for medical oxygen is between 0 and 15 LPM with some units capable of obtaining up to 25 LPM. Many wall flowmeters using a Thorpe tube design are able to be dialed to "flush" oxygen which is beneficial in emergency situations.[citation needed]
Low-dose oxygen
editMany people only require slight increases in inhaled oxygen, rather than pure or near-pure oxygen.[67] These requirements can be met through a number of devices dependent on situation, flow requirements, and personal preference.
A nasal cannula (NC) is a thin tube with two small nozzles inserted into a person's nostrils. It can provide oxygen at low flow rates, 1–6 litres per minute (LPM), delivering an oxygen concentration of 24–40%.[68]
There are also a number of face mask options, such as the simple face mask, often used at between 5 and 10 LPM, capable of delivering oxygen concentrations between 35% and 55%.[68] This is closely related to the more controlled air-entrainment masks, also known as Venturi masks, which can accurately deliver a predetermined oxygen concentration from 24 to 50%.[68]
In some instances, a partial rebreathing mask can be used, which is based on a simple mask, but features a reservoir bag, which can provide oxygen concentrations of 40–70% at 5–15 LPM.[citation needed]
Demand oxygen delivery systems (DODS) or oxygen resuscitators deliver oxygen only when the person inhales or the caregiver presses a button on the mask (e.g., nonbreathing patient).[69] These systems greatly conserve oxygen compared to steady-flow masks, and are useful in emergency situations when a limited supply of oxygen is available and there is a delay in transporting the person to higher care.[69] Due to utilization of a variety of methods for oxygenation requirements performance differences arise.[70] They are very useful in CPR, as the caregiver can deliver rescue breaths composed of 100% oxygen with the press of a button. Care must be taken not to over-inflate the person's lungs, for which some systems employ safety valves. These systems may not be appropriate for people who are unconscious or in respiratory distress because of the required respiratory effort.[citation needed]
High flow oxygen delivery
editFor patients requiring high concentrations of oxygen, a number of devices are available. The most commonly utilized device is the non-rebreather mask (or reservoir mask). Non-rebreather masks draw oxygen from attached reservoir bags with one-way valves that direct exhaled air out of the mask. If flow rate is not sufficient (~10L/min), the bag may collapse on inspiration.[68] This type of mask is indicated for acute medical emergencies. The delivered FIO2 (Inhalation volumetric fraction of molecular oxygen) of this system is 60–80%, depending on oxygen flow and breathing pattern.[71][72]
Another type of device is a humidified high flow nasal cannula which enables flows exceeding a person's peak inspiratory flow demand to be delivered via nasal cannula, thus providing FIO2 of up to 100% because there is no entrainment of room air.[73] This also allows the person to continue to talk, eat, and drink while still receiving therapy.[74] This type of delivery method is associated with greater overall comfort, improved oxygenation, respiratory rates and reduced sputumstatis compared with face mask oxygen.[75][76]
In specialist applications such as aviation, tight-fitting masks can be used. These masks also have applications in anaesthesia, carbon monoxide poisoning treatment and in hyperbaric oxygen therapy.[citation needed]
Positive pressure delivery
editPatients who are unable to breathe on their own will require positive pressure to move oxygen into their lungs for gaseous exchange to take place. Systems for delivery vary in complexity and cost, starting with a basic pocket mask adjunct which can be used to manually deliver artificial respiration with supplemental oxygen delivered through a mask port.
Many emergency medical service members, first aid personnel, and hospital staff may use a bag-valve-mask (BVM), which is a malleable bag attached to a face mask (or invasive airway such as an endotracheal tube or laryngeal mask airway), usually with a reservoir bag attached, which is manually manipulated by the healthcare professional to push oxygen (or air) into the lungs. This is the only procedure allowed for initial treatment of cyanide poisoning in the UK workplace.[77]
Automated versions of the BVM system, known as a resuscitator or pneupac can also deliver measured and timed doses of oxygen directly to people through a facemask or airway. These systems are related to the anaesthetic machines used in operations under general anaesthesia that allow a variable amount of oxygen to be delivered, along with other gases including air, nitrous oxide and inhalational anaesthetics.
Drug delivery
editOxygen and other compressed gases are used in conjunction with a nebulizer to allow delivery of medications to the upper and/or lower airways. Nebulizers use compressed gas to propel liquid medication into therapeutically sized aerosol droplets for deposition to the appropriate portion of the airway. A typical compressed gas flow rate of 8–10 L/min is used to nebulize medications, saline, sterile water, or a combination these treatments into a therapeutic aerosol for inhalation. In the clinical setting, room air (ambient mix of several gasses), molecular oxygen, and Heliox[citation needed] are the most common gases used to nebulize a bolus treatment or a continuous volume of therapeutic aerosols.
Exhalation filters for oxygen masks
editFiltered oxygen masks have the ability to prevent exhaled particles from being released into the surrounding environment. These masks are normally of a closed design such that leaks are minimized and breathing of room air is controlled through a series of one-way valves. Filtration of exhaled breaths is accomplished either by placing a filter on the exhalation port or through an integral filter that is part of the mask itself. These masks first became popular in the Toronto (Canada) healthcare community during the 2003 SARS Crisis. SARS was identified as being respiratory based, and it was determined that conventional oxygen therapy devices were not designed for the containment of exhaled particles.[78][79][80] In 2003, the HiOx80 oxygen mask was released for sale. The HiOx80 mask is a closed design mask that allows a filter to be placed on the exhalation port. Several new designs have emerged in the global healthcare community for the containment and filtration of potentially infectious particles. Other designs include the ISO-O
2 oxygen mask, the Flo2Max oxygen mask, and the O-Mask.
Typical oxygen masks allow a person to breathe in a mixture of room air and therapeutic oxygen. However, as filtered oxygen masks use a closed design that minimizes or eliminates the person's contact with and ability to inhale room air, delivered oxygen concentrations in such devices have been found to be elevated, approaching 99% using adequate oxygen flows.[citation needed] Because all exhaled particles are contained within the mask, nebulized medications are also prevented from releasing into the surrounding atmosphere, decreasing the occupational exposure to healthcare staff and other people.[citation needed]
Aircraft
editIn the United States, most airlines restrict the devices allowed on board an aircraft. As a result, passengers are restricted in what devices they can use. Some airlines will provide cylinders for passengers with an associated fee. Other airlines allow passengers to carry on approved portable concentrators. However, the lists of approved devices varies by airline so passengers may need to check with any airline they are planning to fly on. Passengers are generally not allowed to carry on personal cylinders. In all cases, passengers need to notify the airline in advance of their equipment.
Effective May 13, 2009, the Department of Transportation and FAA ruled that a select number of portable oxygen concentrators are approved for use on all commercial flights.[81] FAA regulations require larger airplanes to carry D-cylinders of oxygen for use in case of an emergency.
Oxygen conserving devices
editSince the 1980s, devices have been available which conserve stored oxygen by delivering it during the portion of the breathing cycle when it is more effectively used. This has the effect of stored oxygen lasting longer, or a smaller, and therefore lighter, portable oxygen delivery system being practicable. This class of device can also be used with portable oxygen concentrators, making them more efficient.[82]
The delivery of supplemental oxygen is most effective if it is made at a point in the breathing cycle when it will be inhaled to the alveoli, where gas transfer occurs. oxygen delivered later in the cycle will be inhaled into physiological dead space, wher it serves no useful purpose as it cannot diffuse into the blood. Oxygen delivered during stages of the breathing cycle in which it is not inhaled is also wasted.[82]
A continuous constant flow rate uses a simple regulator, but is inefficient as a high percentage of the delivered gas does not reach the alveoli, and over half is not inhaled at all. A system which accumulates free-flow oxygen during resting and exhalation stages, (reservoir cannulas) makes a larger part of the oxygen available for inhalation, and it will be selectively inhaled during the initial part of inhalation, which reaches furthest into the lungs. A similar function is provided by a mechanical demand regulator which provides gas only during inhalation, but requires some physical effort by the user, and also ventilates dead space with oxygen. A third class of system (pulse dose oxygen conserving device, or demand pulse devices) senses the start of inhalation and provides a metered bolus, which if correctly matched to requirements, will be sufficient and effectively inhaled into the alveoli.Such systems can be pneumatically or electrically controlled.[82]
Adaptive demand systems[82] A development in pulse demand delivery are devices that automatically adjust the volume of the pulsed bolus to suit the activity level of the user. This adaptive response in intended to reduce desaturation responses caused by exercise rate variation.
Pulsed delivery devices are available as stand alone modules or integrated into a system specifically designed to use compressed gas, liquid oxygen or oxygen concentrator sources. Integrated design usually allows optimisation of the system for the source type at the cost of versatility.[82]
Transtracheal oxygen catheters are inserted directly into the trachea through a small opening in the front of the neck for that purpose. The opening is directed downward, towards the bifurcation of the bronchi. Oxygen introduced through the catheter bypasses the dead spaces of the nose, pharynx and upper trachea during inhalation, and during continuous flow, will accumulate in the anatomic dead space at the end of exhalation and be available for immediate inhalation to the alveoli on the following inhalation. This reduces wastage and provides efficiency roughly three times greater than with external continuous flow. This is roughly equivalent to a reservoir cannula. Transtracheal catheters have been found to be effective during rest, exercise and sleep.[82]
See also
edit- Breathing gas – Gas used for human respiration
- Nebulizer – Drug delivery device
- Mechanical ventilation – Method to mechanically assist or replace spontaneous breathing
- Hyperbaric oxygen therapy – Medical treatment at raised ambient pressure
- Oxygen bar – Establishment that sells oxygen for on-site recreational use
- Emergency medical services – Services providing acute medical care
- Respiratory therapist – Practitioner in cardio-pulmonary medicine
- Oxygen tent – Canopy over a patient to provide supplemental oxygen
- Oxygen firebreak – Safety mechanism designed to extinguish a fire in a medical oxygen delivery tube
- Bottled oxygen (climbing) – Equipment which allows the user to breathe at hypoxic altitudes
- Redento D. Ferranti - Early use of oxygen therapy in the U.S. as an effective approach to rehabilitation for COPD patients.
References
edit- ^ a b c d e f g h British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. pp. 217–218, 302. ISBN 9780857111562.
- ^ a b World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 20. hdl:10665/44053. ISBN 9789241547659.
- ^ Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, eds. (2006). Disease Control Priorities in Developing Countries. World Bank Publications. p. 689. ISBN 9780821361801. Archived from the original on 2017-05-10.
- ^ Macintosh M, Moore T (1999). Caring for the Seriously Ill Patient 2E (2 ed.). CRC Press. p. 57. ISBN 9780340705827. Archived from the original on 2017-01-18.
- ^ Dart RC (2004). Medical Toxicology. Lippincott Williams & Wilkins. pp. 217–219. ISBN 9780781728454. Archived from the original on 2017-01-18.
- ^ Peate I, Wild K, Nair M (2014). Nursing Practice: Knowledge and Care. John Wiley & Sons. p. 572. ISBN 9781118481363. Archived from the original on 2017-01-18.
- ^ a b Martin L (1997). Scuba Diving Explained: Questions and Answers on Physiology and Medical Aspects of Scuba Diving. Lawrence Martin. p. H-1. ISBN 9780941332569. Archived from the original on 2017-01-18.
- ^ a b c d Chu DK, Kim LH, Young PJ, Zamiri N, Almenawer SA, Jaeschke R, et al. (April 2018). "Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis". Lancet. 391 (10131): 1693–1705. doi:10.1016/S0140-6736(18)30479-3. PMID 29726345. S2CID 19162595.
- ^ Agasti TK (2010). Textbook of Anesthesia for Postgraduates. JP Medical Ltd. p. 398. ISBN 9789380704944. Archived from the original on 2017-05-10.
- ^ Rushman GB, Davies NJ, Atkinson RS (1996). A Short History of Anaesthesia: The First 150 Years. Butterworth-Heinemann. p. 39. ISBN 9780750630665. Archived from the original on 2017-05-10.
- ^ a b Wyatt JP, Illingworth RN, Graham CA, Hogg K, Robertson C, Clancy M (2012). Oxford Handbook of Emergency Medicine. OUP Oxford. p. 95. ISBN 9780191016059. Archived from the original on 2017-01-18.
- ^ a b "Clinical Guidelines Update – Oxygen" (PDF). Joint Royal Colleges Ambulance Liaison Committee/Warwick University. April 2009. Archived (PDF) from the original on 2009-07-11. Retrieved 2009-06-29.
- ^ O'Driscoll BR, Howard LS, Davison AG (October 2008). "BTS guideline for emergency oxygen use in adult patients". Thorax. 63 (Suppl 6:vi). British Thoracic Society: vi1-68. doi:10.1136/thx.2008.102947. PMID 18838559.
- ^ a b Siemieniuk RA, Chu DK, Kim LH, Güell-Rous MR, Alhazzani W, Soccal PM, et al. (October 2018). "Oxygen therapy for acutely ill medical patients: a clinical practice guideline". BMJ. 363: k4169. doi:10.1136/bmj.k4169. PMID 30355567. S2CID 53032977.
- ^ a b Brubakk AO, Neuman TS (2003). Bennett and Elliott's physiology and medicine of diving (5th Rev ed.). United States: Saunders Ltd. p. 800. ISBN 0-7020-2571-2.
- ^ Undersea and Hyperbaric Medical Society. "Decompression Sickness or Illness and Arterial Gas Embolism". Archived from the original on 2008-07-05. Retrieved 2008-05-30.
- ^ Acott C (1999). "A brief history of diving and decompression illness". South Pacific Underwater Medicine Society Journal. 29 (2). ISSN 0813-1988. OCLC 16986801. Archived from the original on 2009-02-01. Retrieved 2008-05-30.
{{cite journal}}: CS1 maint: unfit URL (link) - ^ Longphre JM, Denoble PJ, Moon RE, Vann RD, Freiberger JJ (2007). "First aid normobaric oxygen for the treatment of recreational diving injuries". Undersea & Hyperbaric Medicine. 34 (1): 43–9. OCLC 26915585. PMID 17393938. Archived from the original on 2008-06-13. Retrieved 2008-05-30.
{{cite journal}}: CS1 maint: unfit URL (link) - ^ Kol S, Adir Y, Gordon CR, Melamed Y (June 1993). "Oxy-helium treatment of severe spinal decompression sickness after air diving". Undersea & Hyperbaric Medicine. 20 (2): 147–54. PMID 8329941. Archived from the original on 2009-02-01. Retrieved 2008-05-30.
{{cite journal}}: CS1 maint: unfit URL (link) - ^ a b Vincent JL, Taccone FS, He X (2017). "Harmful Effects of Hyperoxia in Postcardiac Arrest, Sepsis, Traumatic Brain Injury, or Stroke: The Importance of Individualized Oxygen Therapy in Critically Ill Patients". Canadian Respiratory Journal. 2017: 2834956. doi:10.1155/2017/2834956. PMC 5299175. PMID 28246487.
- ^ a b American College of Chest Physicians, American Thoracic Society (September 2013), "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation, American College of Chest Physicians and American Thoracic Society, archived from the original on 2013-11-03, retrieved 2013-01-06, which cites
- Croxton TL, Bailey WC (August 2006). "Long-term oxygen treatment in chronic obstructive pulmonary disease: recommendations for future research: an NHLBI workshop report". American Journal of Respiratory and Critical Care Medicine. 174 (4): 373–8. doi:10.1164/rccm.200507-1161WS. PMC 2648117. PMID 16614349.
- O'Driscoll BR, Howard LS, Davison AG (October 2008). "BTS guideline for emergency oxygen use in adult patients". Thorax. 63 (Suppl 6): vi1-68. doi:10.1136/thx.2008.102947. PMID 18838559.
- Macnee W (September 2005). "Prescription of oxygen: still problems after all these years". American Journal of Respiratory and Critical Care Medicine. 172 (5): 517–8. doi:10.1164/rccm.2506007. PMID 16120712.
- ^ McDonald CF, Crockett AJ, Young IH (June 2005). "Adult domiciliary oxygen therapy. Position statement of the Thoracic Society of Australia and New Zealand". The Medical Journal of Australia. 182 (12): 621–6. doi:10.5694/j.1326-5377.2005.tb06848.x. hdl:2440/17207. PMID 15963018. S2CID 1056683.
- ^ Stoller JK, Panos RJ, Krachman S, Doherty DE, Make B (July 2010). "Oxygen therapy for patients with COPD: current evidence and the long-term oxygen treatment trial". Chest. 138 (1): 179–87. doi:10.1378/chest.09-2555. PMC 2897694. PMID 20605816.
- ^ Cranston JM, Crockett AJ, Moss JR, Alpers JH (October 2005). "Domiciliary oxygen for chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 2008 (4). John Wiley & Sons, Ltd: CD001744. doi:10.1002/14651858.cd001744.pub2. PMC 6464709. PMID 16235285.
- ^ Austin MA, Wills KE, Blizzard L, Walters EH, Wood-Baker R (October 2010). "Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial". BMJ. 341 (oct18 2): c5462. doi:10.1136/bmj.c5462. PMC 2957540. PMID 20959284.
- ^ Kim V, Benditt JO, Wise RA, Sharafkhaneh A (May 2008). "Oxygen therapy in chronic obstructive pulmonary disease". Proceedings of the American Thoracic Society. 5 (4): 513–8. doi:10.1513/pats.200708-124ET. PMC 2645328. PMID 18453364.
- ^ Patarinski D (1976). "[Indications and contraindications for oxygen therapy of respiratory insufficiency]". Vutreshni Bolesti (in Bulgarian and English). 15 (4): 44–50. PMID 1007238.
- ^ Agarwal R, Srinivas R, Aggarwal AN, Gupta D (December 2006). "Experience with paraquat poisoning in a respiratory intensive care unit in North India" (PDF). Singapore Medical Journal. 47 (12): 1033–1037. PMID 17139398.
- ^ "EMT Medication Formulary" (PDF). PHECC Clinical Practice Guidelines. Pre-Hospital Emergency Care Council. 15 July 2009. p. 84. Archived from the original (PDF) on 14 May 2011. Retrieved 2010-04-14.
- ^ Knight PR, Kurek C, Davidson BA, Nader ND, Patel A, Sokolowski J, et al. (June 2000). "Acid aspiration increases sensitivity to increased ambient oxygen concentrations". American Journal of Physiology. Lung Cellular and Molecular Physiology. 278 (6): L1240-7. doi:10.1152/ajplung.2000.278.6.L1240. PMID 10835330. S2CID 12450589.
- ^ Nader-Djalal N, Knight PR, Thusu K, Davidson BA, Holm BA, Johnson KJ, Dandona P (July 1998). "Reactive oxygen species contribute to oxygen-related lung injury after acid aspiration". Anesthesia and Analgesia. 87 (1): 127–33. doi:10.1097/00000539-199807000-00028. PMID 9661561. S2CID 19132661.
- ^ Smerz RW (2004). "Incidence of oxygen toxicity during the treatment of dysbarism". Undersea & Hyperbaric Medicine. 31 (2): 199–202. PMID 15485081.
- ^ Hampson NB, Simonson SG, Kramer CC, Piantadosi CA (December 1996). "Central nervous system oxygen toxicity during hyperbaric treatment of patients with carbon monoxide poisoning". Undersea & Hyperbaric Medicine. 23 (4): 215–219. PMID 8989851.
- ^ "Oxygen Therapy". American Cancer Society. 26 December 2012. Archived from the original on 21 March 2012. Retrieved 2013-09-20.
- ^ a b Mach WJ, Thimmesch AR, Pierce JT, Pierce JD (2011-06-05). "Consequences of hyperoxia and the toxicity of oxygen in the lung". Nursing Research and Practice. 2011: 260482. doi:10.1155/2011/260482. PMC 3169834. PMID 21994818.
- ^ a b Hedenstierna G, Edmark L (June 2010). "Mechanisms of atelectasis in the perioperative period". Best Practice & Research. Clinical Anaesthesiology. 24 (2): 157–69. doi:10.1016/j.bpa.2009.12.002. PMID 20608554.
- ^ a b Domino KB (October 2019). "Pre-emergence Oxygenation and Postoperative Atelectasis". Anesthesiology. 131 (4): 771–773. doi:10.1097/ALN.0000000000002875. PMID 31283741. S2CID 195842599.
- ^ Dale WA, Rahn H (September 1952). "Rate of gas absorption during atelectasis". The American Journal of Physiology. 170 (3): 606–13. doi:10.1152/ajplegacy.1952.170.3.606. PMID 12985936.
- ^ O'Brien J (June 2013). "Absorption atelectasis: incidence and clinical implications". AANA Journal. 81 (3): 205–208. PMID 23923671.
- ^ Kallet RH, Matthay MA (January 2013). "Hyperoxic acute lung injury". Respiratory Care. 58 (1): 123–41. doi:10.4187/respcare.01963. PMC 3915523. PMID 23271823.
- ^ Mach WJ, Thimmesch AR, Pierce JT, Pierce JD (2011). "Consequences of hyperoxia and the toxicity of oxygen in the lung". Nursing Research and Practice. 2011: 260482. doi:10.1155/2011/260482. PMC 3169834. PMID 21994818.
- ^ Cooper JS, Phuyal P, Shah N (2021). "Oxygen Toxicity". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 28613494. Retrieved 2021-11-12.
- ^ Cipolla MJ (2009). Control of Cerebral Blood Flow. Morgan & Claypool Life Sciences.
- ^ Sheng M, Liu P, Mao D, Ge Y, Lu H (2017-05-02). "The impact of hyperoxia on brain activity: A resting-state and task-evoked electroencephalography (EEG) study". PLOS ONE. 12 (5): e0176610. Bibcode:2017PLoSO..1276610S. doi:10.1371/journal.pone.0176610. PMC 5412995. PMID 28464001.
- ^ Seo HJ, Bahk WM, Jun TY, Chae JH (2007-02-01). "The Effect of Oxygen Inhalation on Cognitive Function and EEG in Healthy Adults". Clinical Psychopharmacology and Neuroscience. 5 (1): 25–30. ISSN 1738-1088.
- ^ a b c Brugniaux JV, Coombs GB, Barak OF, Dujic Z, Sekhon MS, Ainslie PN (July 2018). "Highs and lows of hyperoxia: physiological, performance, and clinical aspects". American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 315 (1): R1–R27. doi:10.1152/ajpregu.00165.2017. PMID 29488785. S2CID 3634189.
- ^ Tibbles PM, Edelsberg JS (June 1996). "Hyperbaric-oxygen therapy". The New England Journal of Medicine. 334 (25): 1642–8. doi:10.1056/NEJM199606203342506. PMID 8628361.
- ^ Christiansen J, Douglas CG, Haldane JS (July 1914). "The absorption and dissociation of carbon dioxide by human blood". The Journal of Physiology. 48 (4): 244–71. doi:10.1113/jphysiol.1914.sp001659. PMC 1420520. PMID 16993252.
- ^ Hanson CW, Marshall BE, Frasch HF, Marshall C (January 1996). "Causes of hypercarbia with oxygen therapy in patients with chronic obstructive pulmonary disease". Critical Care Medicine. 24 (1): 23–8. doi:10.1097/00003246-199601000-00007. PMID 8565533.
- ^ Waisman D, Brod V, Wolff R, Sabo E, Chernin M, Weintraub Z, et al. (August 2003). "Effects of hyperoxia on local and remote microcirculatory inflammatory response after splanchnic ischemia and reperfusion". American Journal of Physiology. Heart and Circulatory Physiology. 285 (2): H643-52. doi:10.1152/ajpheart.00900.2002. PMID 12714329.
- ^ Riseman JA, Zamboni WA, Curtis A, Graham DR, Konrad HR, Ross DS (November 1990). "Hyperbaric oxygen therapy for necrotizing fasciitis reduces mortality and the need for debridements". Surgery. 108 (5): 847–50. PMID 2237764.
- ^ Heffner JE, Repine JE (August 1989). "Pulmonary strategies of antioxidant defense". The American Review of Respiratory Disease. 140 (2): 531–54. doi:10.1164/ajrccm/140.2.531. PMID 2669581.
- ^ Clark JM, Lambertsen CJ (May 1971). "Rate of development of pulmonary O2 toxicity in man during O2 breathing at 2.0 Ata". Journal of Applied Physiology. 30 (5): 739–52. doi:10.1152/jappl.1971.30.5.739. PMID 4929472.
- ^ a b Kokot M, Kokot F, Franek E, Wiecek A, Nowicki M, Duława J (October 1994). "Effect of isobaric hyperoxemia on erythropoietin secretion in hypertensive patients". Hypertension. 24 (4): 486–90. doi:10.1161/01.HYP.24.4.486. PMID 8088916.
- ^ Sylvester JT, Shimoda LA, Aaronson PI, Ward JP (January 2012). "Hypoxic pulmonary vasoconstriction". Physiological Reviews. 92 (1): 367–520. doi:10.1152/physrev.00041.2010. PMC 9469196. PMID 22298659.
- ^ a b Groves BM, Reeves JT, Sutton JR, Wagner PD, Cymerman A, Malconian MK, et al. (August 1987). "Operation Everest II: elevated high-altitude pulmonary resistance unresponsive to oxygen". Journal of Applied Physiology. 63 (2): 521–30. doi:10.1152/jappl.1987.63.2.521. PMID 3654410.
- ^ a b Day RW (2015). "Comparison between the Acute Pulmonary Vascular Effects of Oxygen with Nitric Oxide and Sildenafil". Frontiers in Pediatrics. 3: 16. doi:10.3389/fped.2015.00016. PMC 4347295. PMID 25785258.
- ^ Mathieu D, Favory R, Collet F, Linke JC, Wattel F (2006). "Physiologic Effects of Hyperbaric Oxygen on Hemodynamics and Microcirculation". Handbook on Hyperbaric Medicine. pp. 75–101. doi:10.1007/1-4020-4448-8_6. ISBN 1-4020-4376-7.
- ^ McNulty PH, King N, Scott S, Hartman G, McCann J, Kozak M, et al. (March 2005). "Effects of supplemental oxygen administration on coronary blood flow in patients undergoing cardiac catheterization". American Journal of Physiology. Heart and Circulatory Physiology. 288 (3): H1057-62. doi:10.1152/ajpheart.00625.2004. PMID 15706043.
- ^ Sands G. "Oxygen Therapy for Headaches". Archived from the original on 2007-12-01. Retrieved 2007-11-26.
- ^ Jain KK (2017). "Physical, Physiological, and Biochemical Aspects of Hyperbaric Oxygenation". Textbook of Hyperbaric Medicine. pp. 11–22. doi:10.1007/978-3-319-47140-2_2. ISBN 978-3-319-47138-9.
- ^ "Luxfer Aluminum Oxygen Cylinders". CPR Savers & First Aid Supply. Archived from the original on 2010-04-18. Retrieved 2010-04-18.
- ^ McCoy R. "Portable Oxygen Concentrators (POC) Performance Variables that Affect Therapy" (PDF). Archived from the original (PDF) on 2007-07-09. Retrieved 2007-07-03.
- ^ "Wilderness & Environmental Medicine: Sage Journals".
- ^ a b c Werley BL, ed. (1991). "Fire Hazards in Oxygen Systems". ASTM Technical Professional training. Philadelphia: ASTM International Subcommittee G-4.05.
- ^ Lindford AJ, Tehrani H, Sassoon EM, O'Neill TJ (June 2006). "Home oxygen therapy and cigarette smoking: a dangerous practice". Annals of Burns and Fire Disasters. 19 (2): 99–100. PMC 3188038. PMID 21991033.
- ^ Kallstrom 2002
- ^ a b c d Hardavella G, Karampinis I, Frille A, Sreter K, Rousalova I (September 2019). "Oxygen devices and delivery systems". Breathe. 15 (3): e108–e116. doi:10.1183/20734735.0204-2019. PMC 6876135. PMID 31777573.
- ^ a b Gloeckl R, Osadnik C, Bies L, Leitl D, Koczulla AR, Kenn K (April 2019). "Comparison of continuous flow versus demand oxygen delivery systems in patients with COPD: A systematic review and meta-analysis". Respirology. 24 (4): 329–337. doi:10.1111/resp.13457. PMID 30556614. S2CID 58768054.
- ^ Bliss PL, McCoy RW, Adams AB (February 2004). "Characteristics of demand oxygen delivery systems: maximum output and setting recommendations". Respiratory Care. 49 (2): 160–165. PMID 14744265.
- ^ Garcia JA, Gardner D, Vines D, Shelledy D, Wettstein R, Peters J (October 2005). "The Oxygen Concentrations Delivered by Different Oxygen Therapy Systems". Chest. 128 (4): 389S–390S. doi:10.1378/chest.128.4_meetingabstracts.389s-b.
- ^ "Earl, John. Delivery of High FiO
2. Cardinal Health Respiratory Abstracts". Archived from the original on 2007-10-20. Retrieved 2020-08-25.{{cite web}}: CS1 maint: bot: original URL status unknown (link) - ^ "What Is Optiflow? Accurate Oxygen Delivery". Fisher & Paykel Healthcare Limited. Archived from the original on 2013-04-03.
- ^ Sim MA, Dean P, Kinsella J, Black R, Carter R, Hughes M (September 2008). "Performance of oxygen delivery devices when the breathing pattern of respiratory failure is simulated". Anaesthesia. 63 (9): 938–40. doi:10.1111/j.1365-2044.2008.05536.x. PMID 18540928. S2CID 205248111.
- ^ Roca O, Riera J, Torres F, Masclans JR (April 2010). "High-flow oxygen therapy in acute respiratory failure". Respiratory Care. 55 (4): 408–13. PMID 20406507. Archived from the original on 2013-05-11.
- ^ Veenstra P, Veeger NJ, Koppers RJ, Duiverman ML, van Geffen WH (2022-10-05). "High-flow nasal cannula oxygen therapy for admitted COPD-patients. A retrospective cohort study". PLOS ONE. 17 (10): e0272372. Bibcode:2022PLoSO..1772372V. doi:10.1371/journal.pone.0272372. PMC 9534431. PMID 36197917.
- ^ "Cyanide poisoning – New recommendations on first aid treatment". Home Health and Safety Executive (HSE). UK Government. Archived from the original on 2009-10-20.
- ^ Hui DS, Hall SD, Chan MT, Chow BK, Ng SS, Gin T, Sung JJ (August 2007). "Exhaled air dispersion during oxygen delivery via a simple oxygen mask". Chest. 132 (2): 540–6. doi:10.1378/chest.07-0636. PMC 7094533. PMID 17573505.
- ^ Mardimae A, Slessarev M, Han J, Sasano H, Sasano N, Azami T, et al. (October 2006). "Modified N95 mask delivers high inspired oxygen concentrations while effectively filtering aerosolized microparticles". Annals of Emergency Medicine. 48 (4): 391–9, 399.e1-2. doi:10.1016/j.annemergmed.2006.06.039. PMC 7118976. PMID 16997675.
- ^ Somogyi R, Vesely AE, Azami T, Preiss D, Fisher J, Correia J, Fowler RA (March 2004). "Dispersal of respiratory droplets with open vs closed oxygen delivery masks: implications for the transmission of severe acute respiratory syndrome". Chest. 125 (3): 1155–7. doi:10.1378/chest.125.3.1155. PMC 7094599. PMID 15006983.
- ^ "FAA Approved Portable Oxygen Concentrators – Positive Testing Results". faa.gov. Archived from the original on 2014-07-02. Retrieved 2014-06-22.
(As of November 2014) Positive Testing Results: AirSep FreeStyle, AirSep LifeStyle, AirSep Focus, AirSep Freestyle 5, (Caire) SeQual eQuinox / Oxywell (model 4000), Delphi RS-00400 / Oxus RS-00400, DeVilbiss Healthcare iGo, Inogen One, Inogen One G2, lnogen One G3, lnova Labs LifeChoice Activox, International Biophysics LifeChoice / lnova Labs LifeChoice, Invacare XPO2, Invacare Solo 2, Oxylife Independence Oxygen Concentrator, Precision Medical EasyPulse, Respironics EverGo, Respironics SimplyGo, Sequal Eclipse, SeQual SAROS, VBox Trooper
- ^ a b c d e f Tiep B, Carter R (2008). "Oxygen conserving devices and methodologies". Chronic Respiratory Disease. 5 (2). crd.sagepub.com: 109–114. doi:10.1177/1479972308090691. PMID 18539725. S2CID 6141420.
Further reading
edit- Kallstrom TJ (June 2002). "AARC Clinical Practice Guideline: oxygen therapy for adults in the acute care facility--2002 revision & update". Respiratory Care. 47 (6): 717–20. PMID 12078655.
- Cahill Lambert AE (November 2005). "Adult domiciliary oxygen therapy: a patient's perspective". The Medical Journal of Australia. 183 (9): 472–3. doi:10.5694/j.1326-5377.2005.tb07125.x. PMID 16274348. S2CID 77689244.