Non-stoichiometric compounds are chemical compounds, almost always solid inorganic compounds, having elemental composition whose proportions cannot be represented by a ratio of small natural numbers (i.e. an empirical formula); most often, in such materials, some small percentage of atoms are missing or too many atoms are packed into an otherwise perfect lattice work.[not verified in body]
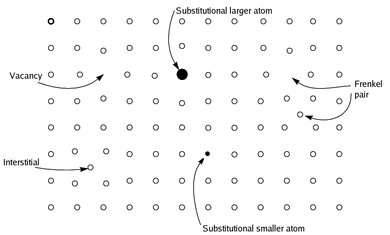
Contrary to earlier definitions, modern understanding of non-stoichiometric compounds view them as homogeneous, and not mixtures of stoichiometric chemical compounds.[not verified in body] Since the solids are overall electrically neutral, the defect is compensated by a change in the charge of other atoms in the solid, either by changing their oxidation state, or by replacing them with atoms of different elements with a different charge. Many metal oxides and sulfides have non-stoichiometric examples; for example, stoichiometric iron(II) oxide, which is rare, has the formula FeO, whereas the more common material is nonstoichiometric, with the formula Fe0.95O. The type of equilibrium defects in non-stoichiometric compounds can vary with attendant variation in bulk properties of the material.[1] Non-stoichiometric compounds also exhibit special electrical or chemical properties because of the defects; for example, when atoms are missing, electrons can move through the solid more rapidly.[not verified in body] Non-stoichiometric compounds have applications in ceramic and superconductive material and in electrochemical (i.e., battery) system designs.[citation needed]
Occurrence
editIron oxides
editNonstoichiometry is pervasive for metal oxides, especially when the metal is not in its highest oxidation state.[2]: 642–644 For example, although wüstite (ferrous oxide) has an ideal (stoichiometric) formula FeO, the actual stoichiometry is closer to Fe0.95O. The non-stoichiometry reflect the ease of oxidation of Fe2+ to Fe3+ effectively replacing a small portion of Fe2+ with two thirds their number of Fe3+. Thus for every three "missing" Fe2+ ions, the crystal contains two Fe3+ ions to balance the charge. The composition of a non-stoichiometric compound usually varies in a continuous manner over a narrow range. Thus, the formula for wüstite is written as Fe1−xO, where x is a small number (0.05 in the previous example) representing the deviation from the "ideal" formula.[3] Nonstoichiometry is especially important in solid, three-dimensional polymers that can tolerate mistakes. To some extent, entropy drives all solids to be non-stoichiometric. But for practical purposes, the term describes materials where the non-stoichiometry is measurable, usually at least 1% of the ideal composition.[citation needed]
Iron sulfides
editThe monosulfides of the transition metals are often nonstoichiometric. Best known perhaps is nominally iron(II) sulfide (the mineral pyrrhotite) with a composition Fe1−xS (x = 0 to 0.2). The rare stoichiometric FeS endmember is known as the mineral troilite. Pyrrhotite is remarkable in that it has numerous polytypes, i.e. crystalline forms differing in symmetry (monoclinic or hexagonal) and composition (Fe7S8, Fe9S10, Fe11S12 and others). These materials are always iron-deficient owing to the presence of lattice defects, namely iron vacancies. Despite those defects, the composition is usually expressed as a ratio of large numbers and the crystals symmetry is relatively high. This means the iron vacancies are not randomly scattered over the crystal, but form certain regular configurations. Those vacancies strongly affect the magnetic properties of pyrrhotite: the magnetism increases with the concentration of vacancies and is absent for the stoichiometric FeS.[4]
Palladium hydrides
editPalladium hydride is a nonstoichiometric material of the approximate composition PdHx (0.02 < x < 0.58). This solid conducts hydrogen by virtue of the mobility of the hydrogen atoms within the solid.[citation needed]
Tungsten oxides
editIt is sometimes difficult to determine if a material is non-stoichiometric or if the formula is best represented by large numbers. The oxides of tungsten illustrate this situation. Starting from the idealized material tungsten trioxide, one can generate a series of related materials that are slightly deficient in oxygen. These oxygen-deficient species can be described as WO3−x, but in fact they are stoichiometric species with large unit cells with the formulas WnO3n−2, where n = 20, 24, 25, 40. Thus, the last species can be described with the stoichiometric formula W40O118, whereas the non-stoichiometric description WO2.95 implies a more random distribution of oxide vacancies.[citation needed]
Other cases
editAt high temperatures (1000 °C), titanium sulfides present a series of non-stoichiometric compounds.[2]: 679
The coordination polymer Prussian blue, nominally Fe7(CN)18 and their analogs are well known to form in non-stoichiometric proportions.[5]: 114 The non-stoichiometric phases exhibit useful properties vis-à-vis their ability to bind caesium and thallium ions.[citation needed]
Applications
editOxidation catalysis
editMany useful compounds are produced by the reactions of hydrocarbons with oxygen, a conversion that is catalyzed by metal oxides. The process operates via the transfer of "lattice" oxygen to the hydrocarbon substrate, a step that temporarily generates a vacancy (or defect). In a subsequent step, the missing oxygen is replenished by O2. Such catalysts rely on the ability of the metal oxide to form phases that are not stoichiometric.[6] An analogous sequence of events describes other kinds of atom-transfer reactions including hydrogenation and hydrodesulfurization catalysed by solid catalysts. These considerations also highlight the fact that stoichiometry is determined by the interior of crystals: the surfaces of crystals often do not follow the stoichiometry of the bulk. The complex structures on surfaces are described by the term "surface reconstruction".
Ion conduction
editThe migration of atoms within a solid is strongly influenced by the defects associated with non-stoichiometry. These defect sites provide pathways for atoms and ions to migrate through the otherwise dense ensemble of atoms that form the crystals. Oxygen sensors and solid state batteries are two applications that rely on oxide vacancies. One example is the CeO2-based sensor in automotive exhaust systems. At low partial pressures of O2, the sensor allows the introduction of increased air to effect more thorough combustion.[6]
Superconductivity
editMany superconductors are non-stoichiometric. For example, yttrium barium copper oxide, arguably the most notable high-temperature superconductor, is a non-stoichiometric solid with the formula YxBa2Cu3O7−x. The critical temperature of the superconductor depends on the exact value of x. The stoichiometric species has x = 0, but this value can be as great as 1.[6]
History
editIt was mainly through the work of Nikolai Semenovich Kurnakov and his students that Berthollet's opposition to Proust's law was shown to have merit for many solid compounds. Kurnakov divided non-stoichiometric compounds into berthollides and daltonides depending on whether their properties showed monotonic behavior with respect to composition or not. The term berthollide was accepted by IUPAC in 1960.[7] The names come from Claude Louis Berthollet and John Dalton, respectively, who in the 19th century advocated rival theories of the composition of substances. Although Dalton "won" for the most part, it was later recognized that the law of definite proportions had important exceptions.[8]
See also
editReferences
edit- ^ Geng, Hua Y.; et al. (2012). "Anomalies in nonstoichiometric uranium dioxide induced by a pseudo phase transition of point defects". Phys. Rev. B. 85 (14): 144111. arXiv:1204.4607. Bibcode:2012PhRvB..85n4111G. doi:10.1103/PhysRevB.85.144111. S2CID 119288531.
- ^ a b N. N. Greenwood & A. Earnshaw, 2012, "Chemistry of the Elements," 2nd Edn., Amsterdam, NH, NLD:Elsevier, ISBN 0080501095, see [1], accessed 8 July 2015. [Page numbers marked by superscript, inline.]
- ^ Lesley E. Smart (2005). Solid State Chemistry: An Introduction, 3rd edition. CRC Press. p. 214. ISBN 978-0-7487-7516-3.
- ^ Hubert Lloyd Barnes (1997). Geochemistry of hydrothermal ore deposits. John Wiley and Sons. pp. 382–390. ISBN 978-0-471-57144-5.
- ^ Metal-Organic and Organic Molecular Magnets Peter Day, Alan E Underhill Royal Society of Chemistry, 2007, ISBN 1847551394, ISBN 9781847551399
- ^ a b c Atkins, P. W.; Overton, T. L.; Rourke, J. P.; Weller, M. T.; Armstrong, F. A., 2010, Shriver and Atkins' Inorganic Chemistry 5th Edn., pp. 65, 75, 99f, 268, 271, 277, 287, 356, 409, Oxford, OXF, GBR: Oxford University Press, ISBN 0199236178, see [2], accessed 8 July 2015.
- ^ The Rare Earth Trifluorides, Part 2 Arxius de les Seccions de Ciències Dmitrii N. Khitarov, Boris Pavlovich Sobolev, Irina V. Alexeeva, Institut d'Estudis Catalans, 2000, p75ff. ISBN 847283610X, ISBN 9788472836105
- ^ Henry Marshall Leicester (1971). The Historical Background of Chemistry. Courier Dover Publications. p. 153. ISBN 9780486610535.
Further reading
edit- F. Albert Cotton, Geoffrey Wilkinson, Carlos A. Murillo & Manfred Bochmann, 1999, Advanced Inorganic Chemistry, 6th Edn., pp. 202, 271, 316, 777, 888. 897, and 1145, New York, NY, USA:Wiley-Interscience, ISBN 0471199575, see [3], accessed 8 July 2015.
- Roland Ward, 1963, Nonstoichiometric Compounds, Advances in Chemistry series, Vol. 39, Washington, DC, USA: American Chemical Society, ISBN 9780841222076, DOI 10.1021/ba-1964-0039, see [4], accessed 8 July 2015.
- J. S. Anderson, 1963, "Current problems in nonstoichiometry (Ch. 1)," in Nonstoichiometric Compounds (Roland Ward, Ed.), pp. 1–22, Advances in Chemistry series, Vol. 39, Washington, DC, USA: American Chemical Society, ISBN 9780841222076, DOI 10.1021/ba-1964-0039.ch001, see [5], accessed 8 July 2015.