The Miller–Urey experiment,[1] or Miller experiment,[2] was an experiment in chemical synthesis carried out in 1952 that simulated the conditions thought at the time to be present in the atmosphere of the early, prebiotic Earth. It is seen as one of the first successful experiments demonstrating the synthesis of organic compounds from inorganic constituents in an origin of life scenario. The experiment used methane (CH4), ammonia (NH3), hydrogen (H2), in ratio 2:2:1, and water (H2O). Applying an electric arc (simulating lightning) resulted in the production of amino acids.
It is regarded as a groundbreaking experiment, and the classic experiment investigating the origin of life (abiogenesis). It was performed in 1952 by Stanley Miller, supervised by Nobel laureate Harold Urey at the University of Chicago, and published the following year. At the time, it supported Alexander Oparin's and J. B. S. Haldane's hypothesis that the conditions on the primitive Earth favored chemical reactions that synthesized complex organic compounds from simpler inorganic precursors.[3][4][5]
After Miller's death in 2007, scientists examining sealed vials preserved from the original experiments were able to show that more amino acids were produced in the original experiment than Miller was able to report with paper chromatography.[6] While evidence suggests that Earth's prebiotic atmosphere might have typically had a composition different from the gas used in the Miller experiment, prebiotic experiments continue to produce racemic mixtures of simple-to-complex organic compounds, including amino acids, under varying conditions.[7] Moreover, researchers have shown that transient, hydrogen-rich atmospheres – conducive to Miller-Urey synthesis – would have occurred after large asteroid impacts on early Earth.[8][9]
History
editFoundations of organic synthesis and the origin of life
editUntil the 19th century, there was considerable acceptance of the theory of spontaneous generation, the idea that "lower" animals, such as insects or rodents, arose from decaying matter.[10] However, several experiments in the 19th century – particularly Louis Pasteur's swan neck flask experiment in 1859[11] — disproved the theory that life arose from decaying matter. Charles Darwin published On the Origin of Species that same year, describing the mechanism of biological evolution.[12] While Darwin never publicly wrote about the first organism in his theory of evolution, in a letter to Joseph Dalton Hooker, he speculated:
But if (and oh what a big if) we could conceive in some warm little pond with all sorts of ammonia and phosphoric salts, light, heat, electricity etcetera present, that a protein compound was chemically formed, ready to undergo still more complex changes [...]"[13]
At this point, it was known that organic molecules could be formed from inorganic starting materials, as Friedrich Wöhler had described Wöhler synthesis of urea from ammonium cyanate in 1828.[14] Several other early seminal works in the field of organic synthesis followed, including Alexander Butlerov's synthesis of sugars from formaldehyde and Adolph Strecker's synthesis of the amino acid alanine from acetaldehyde, ammonia, and hydrogen cyanide.[15] In 1913, Walther Löb synthesized amino acids by exposing formamide to silent electric discharge,[16] so scientists were beginning to produce the building blocks of life from simpler molecules, but these were not intended to simulate any prebiotic scheme or even considered relevant to origin of life questions.[15]
But the scientific literature of the early 20th century contained speculations on the origin of life.[15][17] In 1903, physicist Svante Arrhenius hypothesized that the first microscopic forms of life, driven by the radiation pressure of stars, could have arrived on Earth from space in the panspermia hypothesis.[18] In the 1920s, Leonard Troland wrote about a primordial enzyme that could have formed by chance in the primitive ocean and catalyzed reactions, and Hermann J. Muller suggested that the formation of a gene with catalytic and autoreplicative properties could have set evolution in motion.[19] Around the same time, Alexander Oparin's and J. B. S. Haldane's "Primordial soup" ideas were emerging, which hypothesized that a chemically-reducing atmosphere on early Earth would have been conducive to organic synthesis in the presence of sunlight or lightning, gradually concentrating the ocean with random organic molecules until life emerged.[20] In this way, frameworks for the origin of life were coming together, but at the mid-20th century, hypotheses lacked direct experimental evidence.
Stanley Miller and Harold Urey
editAt the time of the Miller–Urey experiment, Harold Urey was a Professor of Chemistry at the University of Chicago who had a well-renowned career, including receiving the Nobel Prize in Chemistry in 1934 for his isolation of deuterium[21] and leading efforts to use gaseous diffusion for uranium isotope enrichment in support of the Manhattan Project.[22] In 1952, Urey postulated that the high temperatures and energies associated with large impacts in Earth's early history would have provided an atmosphere of methane (CH4), water (H2O), ammonia (NH3), and hydrogen (H2), creating the reducing environment necessary for the Oparin-Haldane "primordial soup" scenario.[23]
Stanley Miller arrived at the University of Chicago in 1951 to pursue a PhD under nuclear physicist Edward Teller, another prominent figure in the Manhattan Project.[24] Miller began to work on how different chemical elements were formed in the early universe, but, after a year of minimal progress, Teller was to leave for California to establish Lawrence Livermore National Laboratory and further nuclear weapons research.[24] Miller, having seen Urey lecture on his 1952 paper, approached him about the possibility of a prebiotic synthesis experiment. While Urey initially discouraged Miller, he agreed to allow Miller to try for a year.[24] By February 1953, Miller had mailed a manuscript as sole author reporting the results of his experiment to Science.[25] Urey refused to be listed on the manuscript because he believed his status would cause others to underappreciate Miller's role in designing and conducting the experiment and so encouraged Miller to take full credit for the work. Despite this the set-up is still most commonly referred to including both their names.[25][26] After not hearing from Science for a few weeks, a furious Urey wrote to the editorial board demanding an answer, stating, "If Science does not wish to publish this promptly we will send it to the Journal of the American Chemical Society."[25] Miller's manuscript was eventually published in Science in May 1953.[25]
Experiment
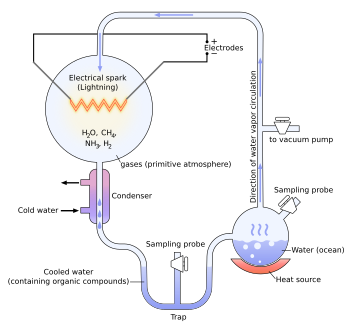
editIn the original 1952 experiment, methane (CH4), ammonia (NH3), and hydrogen (H2) were all sealed together in a 2:2:1 ratio (1 part H2) inside a sterile 5-L glass flask connected to a 500-mL flask half-full of water (H2O). The gas chamber was intended to represent Earth's prebiotic atmosphere, while the water simulated an ocean. The water in the smaller flask was boiled such that water vapor entered the gas chamber and mixed with the "atmosphere". A continuous electrical spark was discharged between a pair of electrodes in the larger flask. The spark passed through the mixture of gases and water vapor, simulating lightning. A condenser below the gas chamber allowed aqueous solution to accumulate into a U-shaped trap at the bottom of the apparatus, which was sampled.
After a day, the solution that had collected at the trap was pink, and after a week of continuous operation the solution was deep red and turbid, which Miller attributed to organic matter adsorbed onto colloidal silica.[3] The boiling flask was then removed, and mercuric chloride (a poison) was added to prevent microbial contamination. The reaction was stopped by adding barium hydroxide and sulfuric acid, and evaporated to remove impurities. Using paper chromatography, Miller identified five amino acids present in the solution: glycine, α-alanine and β-alanine were positively identified, while aspartic acid and α-aminobutyric acid (AABA) were less certain, due to the spots being faint.[3]
Materials and samples from the original experiments remained in 2017 under the care of Miller's former student, Jeffrey Bada, a professor at the UCSD, Scripps Institution of Oceanography who also conducts origin of life research.[27] As of 2013[update], the apparatus used to conduct the experiment was on display at the Denver Museum of Nature and Science.[28]
Chemistry of experiment
editIn 1957 Miller published research describing the chemical processes occurring inside his experiment.[29] Hydrogen cyanide (HCN) and aldehydes (e.g., formaldehyde) were demonstrated to form as intermediates early on in the experiment due to the electric discharge.[29] This agrees with current understanding of atmospheric chemistry, as HCN can generally be produced from reactive radical species in the atmosphere that arise when CH4 and nitrogen break apart under ultraviolet (UV) light.[30] Similarly, aldehydes can be generated in the atmosphere from radicals resulting from CH4 and H2O decomposition and other intermediates like methanol.[31] Several energy sources in planetary atmospheres can induce these dissociation reactions and subsequent hydrogen cyanide or aldehyde formation, including lightning,[32] ultraviolet light,[30] and galactic cosmic rays.[33]
For example, here is a set photochemical reactions of species in the Miller-Urey atmosphere that can result in formaldehyde:[31]
- H2O + hv → H + OH[34]
- CH4 + OH → CH3 + HOH[35]
- CH3 + OH → CH3OH[36]
- CH3OH + hv → CH2O (formaldehyde) + H2[37]
A photochemical path to HCN from NH3 and CH4 is:[39]
- NH3 + hv → NH2 + H
- NH2 + CH4 → NH3 + CH3
- NH2 + CH3 → CH5N
- CH5N + hv → HCN + 2H2
Other active intermediate compounds (acetylene, cyanoacetylene, etc.) have been detected in the aqueous solution of Miller–Urey-type experiments,[40] but the immediate HCN and aldehyde production, the production of amino acids accompanying the plateau in HCN and aldehyde concentrations, and slowing of amino acid production rate during HCN and aldehyde depletion provided strong evidence that Strecker amino acid synthesis was occurring in the aqueous solution.[29]
Strecker synthesis describes the reaction of an aldehyde, ammonia, and HCN to a simple amino acid through an aminoacetonitrile intermediate:
- CH2O + HCN + NH3 → NH2-CH2-CN (aminoacetonitrile) + H2O
- NH2-CH2-CN + 2H2O → NH3 + NH2-CH2-COOH (glycine)
Furthermore, water and formaldehyde can react via Butlerov's reaction to produce various sugars like ribose.[41]
The experiments showed that simple organic compounds, including the building blocks of proteins and other macromolecules, can abiotically be formed from gases with the addition of energy.
Related experiments and follow-up work
editContemporary experiments
editThere were a few similar spark discharge experiments contemporaneous with Miller-Urey. An article in The New York Times (March 8, 1953) titled "Looking Back Two Billion Years" describes the work of Wollman M. MacNevin at Ohio State University, before the Miller Science paper was published in May 1953. MacNevin was passing 100,000V sparks through methane and water vapor and produced "resinous solids" that were "too complex for analysis."[25][42][43] Furthermore, K. A. Wilde submitted a manuscript to Science on December 15, 1952, before Miller submitted his paper to the same journal in February 1953. Wilde's work, published on July 10, 1953, used voltages up to only 600V on a binary mixture of carbon dioxide (CO2) and water in a flow system and did not note any significant reduction products.[44] According to some, the reports of these experiments explain why Urey was rushing Miller's manuscript through Science and threatening to submit to the Journal of the American Chemical Society.[25]
By introducing an experimental framework to test prebiotic chemistry, the Miller–Urey experiment paved the way for future origin of life research.[45] In 1961, Joan Oró produced milligrams of the nucleobase adenine from a concentrated solution of HCN and NH3 in water.[46] Oró found that several amino acids were also formed from HCN and ammonia under those conditions.[47] Experiments conducted later showed that the other RNA and DNA nucleobases could be obtained through simulated prebiotic chemistry with a reducing atmosphere.[48][49] Other researchers also began using UV-photolysis in prebiotic schemes, as the UV flux would have been much higher on early Earth.[50] For example, UV-photolysis of water vapor with carbon monoxide was found to yield various alcohols, aldehydes, and organic acids.[51] In the 1970s, Carl Sagan used Miller-Urey-type reactions to synthesize and experiment with complex organic particles dubbed "tholins", which likely resemble particles formed in hazy atmospheres like that of Titan.[52]
Modified Miller–Urey experiments
editMuch work has been done since the 1950s toward understanding how Miller-Urey chemistry behaves in various environmental settings. In 1983, testing different atmospheric compositions, Miller and another researcher repeated experiments with varying proportions of H2, H2O, N2, CO2 or CH4, and sometimes NH3.[53] They found that the presence or absence of NH3 in the mixture did not significantly impact amino acid yield, as NH3 was generated from N2 during the spark discharge.[53] Additionally, CH4 proved to be one of the most important atmospheric ingredients for high yields, likely due to its role in HCN formation.[53] Much lower yields were obtained with more oxidized carbon species in place of CH4, but similar yields could be reached with a high H2/CO2 ratio.[53] Thus, Miller-Urey reactions work in atmospheres of other compositions as well, depending on the ratio of reducing and oxidizing gases. More recently, Jeffrey Bada and H. James Cleaves, graduate students of Miller, hypothesized that the production of nitrites, which destroy amino acids, in CO2 and N2-rich atmospheres may explain low amino acids yields.[54] In a Miller-Urey setup with a less-reducing (CO2 + N2 + H2O) atmosphere, when they added calcium carbonate to buffer the aqueous solution and ascorbic acid to inhibit oxidation, yields of amino acids greatly increased, demonstrating that amino acids can still be formed in more neutral atmospheres under the right geochemical conditions.[54] In a prebiotic context, they argued that seawater would likely still be buffered and ferrous iron could inhibit oxidation.[54]
In 1999, after Miller suffered a stroke, he donated the contents of his laboratory to Bada.[27] In an old cardboard box, Bada discovered unanalyzed samples from modified experiments that Miller had conducted in the 1950s.[27] In a "volcanic" apparatus, Miller had amended an aspirating nozzle to shoot a jet of steam into the reaction chamber.[7][55] Using high-performance liquid chromatography and mass spectrometry, Bada's lab analyzed old samples from a set of experiments Miller conducted with this apparatus and found some higher yields and a more diverse suite of amino acids.[7][55] Bada speculated that injecting the steam into the spark could have split water into H and OH radicals, leading to more hydroxylated amino acids during Strecker synthesis.[7][55] In a separate set of experiments, Miller added hydrogen sulfide (H2S) to the reducing atmosphere, and Bada's analyses of the products suggested order-of-magnitude higher yields, including some amino acids with sulfur moieties.[7][56]
A 2021 work highlighted the importance of the high-energy free electrons present in the experiment. It is these electrons that produce ions and radicals, and represent an aspect of the experiment that needs to be better understood.[57]
After comparing Miller–Urey experiments conducted in borosilicate glassware with those conducted in Teflon apparatuses, a 2021 paper suggests that the glass reaction vessel acts as a mineral catalyst, implicating silicate rocks as important surfaces in prebiotic Miller-Urey reactions.[58]
Early Earth's prebiotic atmosphere
editWhile there is a lack of geochemical observations to constrain the exact composition of the prebiotic atmosphere, recent models point to an early "weakly reducing" atmosphere; that is, early Earth's atmosphere was likely dominated by CO2 and N2 and not CH4 and NH3 as used in the original Miller–Urey experiment.[59][60] This is explained, in part, by the chemical composition of volcanic outgassing. Geologist William Rubey was one of the first to compile data on gases emitted from modern volcanoes and concluded that they are rich in CO2, H2O, and likely N2, with varying amounts of H2, sulfur dioxide (SO2), and H2S.[60][61] Therefore, if the redox state of Earth's mantle — which dictates the composition of outgassing – has been constant since formation, then the atmosphere of early Earth was likely weakly reducing, but there are some arguments for a more-reducing atmosphere for the first few hundred million years.[60]
While the prebiotic atmosphere could have had a different redox condition than that of the Miller–Urey atmosphere, the modified Miller–Urey experiments described in the above section demonstrated that amino acids can still be abiotically produced in less-reducing atmospheres under specific geochemical conditions.[7][53][54] Furthermore, harkening back to Urey's original hypothesis of a "post-impact" reducing atmosphere,[23] a recent atmospheric modeling study has shown that an iron-rich impactor with a minimum mass around 4×1020 – 5×1021 kg would be enough to transiently reduce the entire prebiotic atmosphere, resulting in a Miller-Urey-esque H2-, CH4-, and NH3-dominated atmosphere that persists for millions of years.[9] Previous work has estimated from the lunar cratering record and composition of Earth's mantle that between four and seven such impactors reached the Hadean Earth.[8][9][62]
A large factor controlling the redox budget of early Earth's atmosphere is the rate of atmospheric escape of H2 after Earth's formation. Atmospheric escape – common to young, rocky planets — occurs when gases in the atmosphere have sufficient kinetic energy to overcome gravitational energy.[63] It is generally accepted that the timescale of hydrogen escape is short enough such that H2 made up < 1% of the atmosphere of prebiotic Earth,[60] but, in 2005, a hydrodynamic model of hydrogen escape predicted escape rates two orders of magnitude lower than previously thought, maintaining a hydrogen mixing ratio of 30%.[64] A hydrogen-rich prebiotic atmosphere would have large implications for Miller-Urey synthesis in the Hadean and Archean, but later work suggests solutions in that model might have violated conservation of mass and energy.[63][65] That said, during hydrodynamic escape, lighter molecules like hydrogen can "drag" heavier molecules with them through collisions, and recent modeling of xenon escape has pointed to a hydrogen atmospheric mixing ratio of at least 1% or higher at times during the Archean.[66]
Taken together, the view that early Earth's atmosphere was weakly reducing, with transient instances of highly-reducing compositions following large impacts is generally supported.[9][23][60]
Extraterrestrial sources of amino acids
editConditions similar to those of the Miller–Urey experiments are present in other regions of the Solar System, often substituting ultraviolet light for lightning as the energy source for chemical reactions.[67][68][69] The Murchison meteorite that fell near Murchison, Victoria, Australia in 1969 was found to contain an amino acid distribution remarkably similar to Miller-Urey discharge products.[27] Analysis of the organic fraction of the Murchison meteorite with Fourier-transform ion cyclotron resonance mass spectrometry detected over 10,000 unique compounds,[70] albeit at very low (ppb–ppm) concentrations.[71][72] In this way, the organic composition of the Murchison meteorite is seen as evidence of Miller-Urey synthesis outside Earth.
Comets and other icy outer-solar-system bodies are thought to contain large amounts of complex carbon compounds (such as tholins) formed by processes akin to Miller-Urey setups, darkening surfaces of these bodies.[52][73] Some argue that comets bombarding the early Earth could have provided a large supply of complex organic molecules along with the water and other volatiles,[74][75] however very low concentrations of biologically-relevant material combined with uncertainty surrounding the survival of organic matter upon impact make this difficult to determine.[15]
Relevance to the origin of life
editThe Miller–Urey experiment was proof that the building blocks of life could be synthesized abiotically from gases, and introduced a new prebiotic chemistry framework through which to study the origin of life. Simulations of protein sequences present in the last universal common ancestor (LUCA), or the last shared ancestor of all extant species today, show an enrichment in simple amino acids that were available in the prebiotic environment according to Miller-Urey chemistry. This suggests that the genetic code from which all life evolved was rooted in a smaller suite of amino acids than those used today.[76] Thus, while creationist arguments focus on the fact that Miller–Urey experiments have not generated all 22 genetically-encoded amino acids,[77] this does not actually conflict with the evolutionary perspective on the origin of life.[76]
Another common criticism is that the racemic (containing both L and D enantiomers) mixture of amino acids produced in a Miller–Urey experiment is not exemplary of abiogenesis theories,[77] as life on Earth today uses almost exclusively L-amino acids.[79] While it is true that Miller-Urey setups produce racemic mixtures,[80] the origin of homochirality is a separate area in origin of life research.[81]
Recent work demonstrates that magnetic mineral surfaces like magnetite can be templates for the enantioselective crystallization of chiral molecules, including RNA precursors, due to the chiral-induced spin selectivity (CISS) effect.[82][83] Once an enantioselective bias is introduced, homochirality can then propagate through biological systems in various ways.[84] In this way, enantioselective synthesis is not required of Miller-Urey reactions if other geochemical processes in the environment are introducing homochirality.
Finally, Miller-Urey and similar experiments primarily deal with the synthesis of monomers; polymerization of these building blocks to form peptides and other more complex structures is the next step of prebiotic chemistry schemes.[85] Polymerization requires condensation reactions, which are thermodynamically unfavored in aqueous solutions because they expel water molecules.[86] Scientists as far back as John Desmond Bernal in the late 1940s thus speculated that clay surfaces would play a large role in abiogenesis, as they might concentrate monomers.[87] Several such models for mineral-mediated polymerization have emerged, such as the interlayers of layered double hydroxides like green rust over wet-dry cycles.[88] Some scenarios for peptide formation have been proposed that are even compatible with aqueous solutions, such as the hydrophobic air-water interface[86] and a novel "sulfide-mediated α-aminonitrile ligation" scheme, where amino acid precursors come together to form peptides.[89] Polymerization of life's building blocks is an active area of research in prebiotic chemistry.
Amino acids identified
editBelow is a table of amino acids produced and identified in the "classic" 1952 experiment, as analyzed by Miller in 1952[3] and more recently by Bada and collaborators with modern mass spectrometry,[7] the 2008 re-analysis of vials from the volcanic spark discharge experiment,[7][55] and the 2010 re-analysis of vials from the H2S-rich spark discharge experiment.[7][56] While not all proteinogenic amino acids have been produced in spark discharge experiments, it is generally accepted that early life used a simpler set of prebiotically-available amino acids.[76]
| Amino acid | Produced in experiment | Proteinogenic | |||
|---|---|---|---|---|---|
| Miller–Urey (1952) |
Bada Reanalysis of 1950s product (2008–) |
Volcanic spark discharge (2008) |
H2S-rich spark discharge (2010) | ||
| Glycine | Yes | ||||
| α-Alanine | Yes | ||||
| β-Alanine | No | ||||
| Aspartic acid | Yes | ||||
| α-Aminobutyric acid | No | ||||
| Serine | Yes | ||||
| Isoserine | No | ||||
| α-Aminoisobutyric acid | No | ||||
| β-Aminoisobutyric acid | No | ||||
| β-Aminobutyric acid | No | ||||
| γ-Aminobutyric acid | No | ||||
| Valine | Yes | ||||
| Isovaline | No | ||||
| Glutamic acid | Yes | ||||
| Norvaline | No | ||||
| Methylamine | No | ||||
| Ethylamine | No | ||||
| Ethanolamine | No | ||||
| Isopropylamine | No | ||||
| n-Propylamine | No | ||||
| α-Aminoadipic acid | No | ||||
| Homoserine | No | ||||
| 2-Methylserine | No | ||||
| β-Hydroxyaspartic acid | No | ||||
| Ornithine | No | ||||
| 2-Methylglutamic acid | No | ||||
| Phenylalanine | Yes | ||||
| Homocysteic acid | No | ||||
| S-Methylcysteine | No | ||||
| Methionine | Yes | ||||
| Methionine sulfoxide | No | ||||
| Methionine sulfone | No | ||||
| Isoleucine | Yes | ||||
| Leucine | Yes | ||||
| Ethionine | No | ||||
| Cysteine | Yes | ||||
| Histidine | Yes | ||||
| Lysine | Yes | ||||
| Asparagine | Yes | ||||
| Pyrrolysine | Yes | ||||
| Proline | Yes | ||||
| Glutamine | Yes | ||||
| Arginine | Yes | ||||
| Threonine | Yes | ||||
| Selenocysteine | Yes | ||||
| Tryptophan | Yes | ||||
| Tyrosine | Yes | ||||
References
edit- ^ Hill HG, Nuth JA (2003). "The catalytic potential of cosmic dust: implications for prebiotic chemistry in the solar nebula and other protoplanetary systems". Astrobiology. 3 (2): 291–304. Bibcode:2003AsBio...3..291H. doi:10.1089/153110703769016389. PMID 14577878.
- ^ Balm SP; Hare J.P.; Kroto HW (1991). "The analysis of comet mass spectrometric data". Space Science Reviews. 56 (1–2): 185–9. Bibcode:1991SSRv...56..185B. doi:10.1007/BF00178408. S2CID 123124418.
- ^ a b c d Miller, Stanley L. (1953). "Production of Amino Acids Under Possible Primitive Earth Conditions" (PDF). Science. 117 (3046): 528–9. Bibcode:1953Sci...117..528M. doi:10.1126/science.117.3046.528. PMID 13056598. Archived from the original (PDF) on March 17, 2012. Retrieved January 17, 2011.
- ^ Miller, Stanley L.; Harold C. Urey (1959). "Organic Compound Synthesis on the Primitive Earth". Science. 130 (3370): 245–51. Bibcode:1959Sci...130..245M. doi:10.1126/science.130.3370.245. PMID 13668555. Miller states that he made "A more complete analysis of the products" in the 1953 experiment, listing additional results.
- ^ A. Lazcano; J. L. Bada (2004). "The 1953 Stanley L. Miller Experiment: Fifty Years of Prebiotic Organic Chemistry". Origins of Life and Evolution of Biospheres. 33 (3): 235–242. Bibcode:2003OLEB...33..235L. doi:10.1023/A:1024807125069. PMID 14515862. S2CID 19515024.
- ^ "The Spark of Life". BBC Four. August 26, 2009. Archived from the original on November 13, 2010. TV Documentary.
{{cite web}}: CS1 maint: postscript (link) - ^ a b c d e f g h i Bada, Jeffrey L. (2013). "New insights into prebiotic chemistry from Stanley Miller's spark discharge experiments". Chemical Society Reviews. 42 (5): 2186–96. doi:10.1039/c3cs35433d. PMID 23340907. S2CID 12230177.
- ^ a b Zahnle, Kevin J.; Lupu, Roxana; Catling, David C.; Wogan, Nick (May 1, 2020). "Creation and Evolution of Impact-generated Reduced Atmospheres of Early Earth". The Planetary Science Journal. 1 (1): 11. arXiv:2001.00095. Bibcode:2020PSJ.....1...11Z. doi:10.3847/PSJ/ab7e2c. ISSN 2632-3338.
- ^ a b c d e Wogan, Nicholas F.; Catling, David C.; Zahnle, Kevin J.; Lupu, Roxana (September 1, 2023). "Origin-of-life Molecules in the Atmosphere after Big Impacts on the Early Earth". The Planetary Science Journal. 4 (9): 169. arXiv:2307.09761. Bibcode:2023PSJ.....4..169W. doi:10.3847/psj/aced83. ISSN 2632-3338.
- ^ Sheldon, Robert B. (August 18, 2005). "Historical development of the distinction between bio- and abiogenesis". In Hoover, Richard B.; Levin, Gilbert V.; Rozanov, Alexei Y.; Gladstone, G. Randall (eds.). Astrobiology and Planetary Missions. Vol. 5906. pp. 444–456. doi:10.1117/12.663480. S2CID 44194609.
- ^ "Pasteur's "col de cygnet" (1859) | British Society for Immunology". May 27, 2022. Archived from the original on May 27, 2022. Retrieved November 11, 2023.
- ^ Darwin, Charles (1859). On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. London: John Murray.
- ^ Darwin, Charles. Darwin Correspondence Project, "Letter No. 7471", 1871. Available online: http://www.darwinproject.ac.uk/DCP-LETT-7471
- ^ Friedrich Wöhler (1828). "Ueber künstliche Bildung des Harnstoffs". Annalen der Physik und Chemie. 88 (2): 253–256
- ^ a b c d Miller, S. L., & Cleaves, H. J. (2006). Prebiotic chemistry on the primitive Earth. Systems biology, 1, 1.
- ^ Löb, W. (1913). Über das Verhalten des Formamids unter der Wirkung der stillen Entladung Ein Beitrag zur Frage der Stickstoff‐Assimilation. Berichte der deutschen chemischen Gesellschaft, 46(1), 684–697.
- ^ Schopf, J. William, ed. (2002). Life's origin: the beginnings of biological evolution. Berkeley, Calif.: Univ. of California Press. ISBN 978-0-520-23391-1.
- ^ Arrhenius, Svante (1903). "Die Verbreitung des Lebens im Weltenraum" [The Distribution of Life in Space]. Die Umschau.
- ^ Lazcano, A. (November 1, 2010). "Historical Development of Origins Research". Cold Spring Harbor Perspectives in Biology. 2 (11): a002089. doi:10.1101/cshperspect.a002089. ISSN 1943-0264. PMC 2964185. PMID 20534710.
- ^ Kumar, Dhavendra; Steele, Edward J.; Wickramasinghe, N. Chandra (2020), Preface: The origin of life and astrobiology, Advances in Genetics, vol. 106, Elsevier, pp. xv–xviii, doi:10.1016/s0065-2660(20)30037-7, ISBN 978-0-12-821518-0, PMC 7568464, PMID 33081930
- ^ Harold C. Urey – Biographical. NobelPrize.org. Nobel Prize Outreach AB 2023. Mon. 13 Nov 2023. https://www.nobelprize.org/prizes/chemistry/1934/urey/biographical/
- ^ "Harold C. Urey". www.nndb.com. Retrieved November 13, 2023.
- ^ a b c Urey, Harold C. (April 1, 1952). "On the Early Chemical History of the Earth and the Origin of Life". Proceedings of the National Academy of Sciences. 38 (4): 351–363. Bibcode:1952PNAS...38..351U. doi:10.1073/pnas.38.4.351. PMC 1063561. PMID 16589104.
- ^ a b c Lazcano, Antonio; Bada, Jeffrey L. (October 1, 2008). "Stanley L. Miller (1930–2007): Reflections and Remembrances". Origins of Life and Evolution of Biospheres. 38 (5): 373–381. Bibcode:2008OLEB...38..373L. doi:10.1007/s11084-008-9145-2. ISSN 1573-0875. PMID 18726708. S2CID 1167340.
- ^ a b c d e f Lazcano, Antonio; Bada, Jeffrey L. (June 1, 2003). "The 1953 Stanley L. Miller Experiment: Fifty Years of Prebiotic Organic Chemistry". Origins of Life and Evolution of the Biosphere. 33 (3): 235–242. Bibcode:2003OLEB...33..235L. doi:10.1023/A:1024807125069. ISSN 1573-0875. PMID 14515862.
- ^ Marshall, Michael (November 6, 2023). "The elusive origins of life". New Scientist Magazine. Australia: New Scientist.
- ^ a b c d Dreifus, Claudia (May 17, 2010). "A Conversation With Jeffrey L. Bada: A Marine Chemist Studies How Life Began". nytimes.com. Archived from the original on January 18, 2017.
- ^ "Astrobiology Collection: Miller-Urey Apparatus". Denver Museum of Nature & Science. Archived from the original on May 24, 2013.
- ^ a b c d Miller, Stanley L. (January 1, 1957). "The mechanism of synthesis of amino acids by electric discharges". Biochimica et Biophysica Acta. 23 (3): 480–489. doi:10.1016/0006-3002(57)90366-9. ISSN 0006-3002. PMID 13426157.
- ^ a b Sullivan, Woodruff Turner; Baross, John A. (2007). Planets and life: the emerging science of astrobiology. Cambridge: Cambridge University Press. ISBN 978-0-521-82421-7.
- ^ a b Ferris, J. P.; Chen, C. T. (1975). "Chemical evolution. XXVI. Photochemistry of methane, nitrogen, and water mixtures as a model for the atmosphere of the primitive earth". Journal of the American Chemical Society. 97 (11): 2962–2967. doi:10.1021/ja00844a007. ISSN 0002-7863. PMID 1133344.
- ^ Rimmer, P. B.; Helling, Ch (May 23, 2016). "A Chemical Kinetics Network for Lightning and Life in Planetary Atmospheres". The Astrophysical Journal Supplement Series. 224 (1): 9. arXiv:1510.07052. Bibcode:2016ApJS..224....9R. doi:10.3847/0067-0049/224/1/9. ISSN 1538-4365.
- ^ Huntress, W. T. (1976). "The chemistry of planetary atmospheres". Journal of Chemical Education. 53 (4): 204. Bibcode:1976JChEd..53..204H. doi:10.1021/ed053p204. ISSN 0021-9584.
- ^ Getoff, N.; Schenck, G. O. (1968). "PRIMARY PRODUCTS OF LIQUID WATER PHOTOLYSIS AT 1236, 1470 AND 1849 Å". Photochemistry and Photobiology. 8 (3): 167–178. doi:10.1111/j.1751-1097.1968.tb05859.x. ISSN 0031-8655. S2CID 97474816.
- ^ Wilson, Wm. E. (April 1, 1972). "A Critical Review of the Gas-Phase Reaction Kinetics of the Hydroxyl Radical". Journal of Physical and Chemical Reference Data. 1 (2): 535–573. Bibcode:1972JPCRD...1..535W. doi:10.1063/1.3253102. ISSN 0047-2689.
- ^ Greenberg, Raymond I.; Heicklen, Julian (1972). "The reaction of O( 1 D ) with CH 4". International Journal of Chemical Kinetics. 4 (4): 417–432. doi:10.1002/kin.550040406. ISSN 0538-8066.
- ^ Hagege, Janine; Leach, Sydney; Vermeil, Catherine (1965). "Photochimie du méthanol en phase vapeur A 1 236 et A 1 849 Å". Journal de Chimie Physique (in French). 62: 736–746. Bibcode:1965JCP....62..736H. doi:10.1051/jcp/1965620736. ISSN 0021-7689.
- ^ Cleaves, H. James (2012). "Prebiotic Chemistry: What We Know, What We Don't". Evolution: Education and Outreach. 5 (3): 342–360. doi:10.1007/s12052-012-0443-9. ISSN 1936-6434.
- ^ Hu, Renyu (November 1, 2021). "Photochemistry and Spectral Characterization of Temperate and Gas-rich Exoplanets". The Astrophysical Journal. 921 (1): 27. arXiv:2108.04419. Bibcode:2021ApJ...921...27H. doi:10.3847/1538-4357/ac1789. ISSN 0004-637X.
- ^ Orgel, Leslie E. (2004). "Prebiotic Adenine Revisited: Eutectics and Photochemistry". Origins of Life and Evolution of the Biosphere. 34 (4): 361–369. Bibcode:2004OLEB...34..361O. doi:10.1023/B:ORIG.0000029882.52156.c2. PMID 15279171. S2CID 4998122.
- ^ Mense, Thorben H. (December 2019). "A Closer Look at Reactions in the Miller-Urey-Experiment using Coupled Gas Chromatography – Mass Spectrometry" (PDF). Bielefeld University.
- ^ Krehl, Peter O. K. (2009). History of Shock Waves, Explosions and Impact: A Chronological and Biographical Reference. Springer-Verlag. p. 603.
- ^ "Looking Back Two Billion Years". The New York Times. Retrieved November 14, 2023.
- ^ Wilde, Kenneth A.; Zwolinski, Bruno J.; Parlin, Ransom B. (July 1953). "The Reaction Occurring in CO2, 2O Mixtures in a High-Frequency Electric Arc". Science. 118 (3054): 43–44. Bibcode:1953Sci...118...43W. doi:10.1126/science.118.3054.43-a. PMID 13076175. S2CID 11170339.
- ^ James Cleaves II, H. (2022). "The Miller–Urey Experiment's Impact on Modern Approaches to Prebiotic Chemistry". Prebiotic Chemistry and Life's Origin: 165–176. doi:10.1039/9781839164798-00165. ISBN 978-1-78801-749-7.
- ^ Oró J, Kimball AP (August 1961). "Synthesis of purines under possible primitive earth conditions. I. Adenine from hydrogen cyanide". Archives of Biochemistry and Biophysics. 94 (2): 217–27. doi:10.1016/0003-9861(61)90033-9. PMID 13731263.
- ^ Oró J, Kamat SS (April 1961). "Amino-acid synthesis from hydrogen cyanide under possible primitive earth conditions". Nature. 190 (4774): 442–3. Bibcode:1961Natur.190..442O. doi:10.1038/190442a0. PMID 13731262. S2CID 4219284.
- ^ Oró J (1967). Fox SW (ed.). Origins of Prebiological Systems and of Their Molecular Matrices. New York Academic Press. p. 137.
- ^ Ferus, Martin; Pietrucci, Fabio; Saitta, Antonino Marco; Knížek, Antonín; Kubelík, Petr; Ivanek, Ondřej; Shestivska, Violetta; Civiš, Svatopluk (April 25, 2017). "Formation of nucleobases in a Miller–Urey reducing atmosphere". Proceedings of the National Academy of Sciences. 114 (17): 4306–4311. Bibcode:2017PNAS..114.4306F. doi:10.1073/pnas.1700010114. ISSN 0027-8424. PMC 5410828. PMID 28396441.
- ^ Canuto, V. M.; Levine, J. S.; Augustsson, T. R.; Imhoff, C. L. (June 1, 1983). "Oxygen and ozone in the early Earth's atmosphere". Precambrian Research. Development and interactions of the Precambrian atmosphere, lithosphere and biosphere: results and challenges. 20 (2): 109–120. Bibcode:1983PreR...20..109C. doi:10.1016/0301-9268(83)90068-2. ISSN 0301-9268.
- ^ Bar-Nun, Akiva; Hartman, Hyman (1978). "Synthesis of organic compounds from carbon monoxide and water by UV photolysis". Origins of Life. 9 (2): 93–101. Bibcode:1978OrLi....9...93B. doi:10.1007/BF00931407. PMID 752138. S2CID 33972427.
- ^ a b Sagan, Carl; Khare, B. N. (1979). "Tholins: organic chemistry of interstellar grains and gas". Nature. 277 (5692): 102–107. Bibcode:1979Natur.277..102S. doi:10.1038/277102a0. ISSN 1476-4687. S2CID 4261076.
- ^ a b c d e Miller, Stanley L.; Schlesinger, Gordon (January 1, 1983). "The atmosphere of the primitive earth and the prebiotic synthesis of organic compounds". Advances in Space Research. 3 (9): 47–53. Bibcode:1983AdSpR...3i..47M. doi:10.1016/0273-1177(83)90040-6. ISSN 0273-1177. PMID 11542461.
- ^ a b c d Cleaves, H. James; Chalmers, John H.; Lazcano, Antonio; Miller, Stanley L.; Bada, Jeffrey L. (2008). "A Reassessment of Prebiotic Organic Synthesis in Neutral Planetary Atmospheres". Origins of Life and Evolution of Biospheres. 38 (2): 105–115. Bibcode:2008OLEB...38..105C. doi:10.1007/s11084-007-9120-3. ISSN 0169-6149. PMID 18204914. S2CID 7731172.
- ^ a b c d Johnson, Adam P.; Cleaves, H. James; Dworkin, Jason P.; Glavin, Daniel P.; Lazcano, Antonio; Bada, Jeffrey L. (October 17, 2008). "The Miller Volcanic Spark Discharge Experiment". Science. 322 (5900): 404. Bibcode:2008Sci...322..404J. doi:10.1126/science.1161527. ISSN 0036-8075. PMID 18927386. S2CID 10134423.
- ^ a b Parker, Eric T.; Cleaves, Henderson J.; Dworkin, Jason P.; Glavin, Daniel P.; Callahan, Michael; Aubrey, Andrew; Lazcano, Antonio; Bada, Jeffrey L. (April 5, 2011). "Primordial synthesis of amines and amino acids in a 1958 Miller H 2 S-rich spark discharge experiment". Proceedings of the National Academy of Sciences. 108 (14): 5526–5531. doi:10.1073/pnas.1019191108. ISSN 0027-8424. PMC 3078417. PMID 21422282.
- ^ Micca Longo, Gaia; Vialetto, Luca; Diomede, Paola; Longo, Savino; Laporta, Vincenzo (June 16, 2021). "Plasma modeling and prebiotic chemistry: A review of the state-of-the-art and perspectives". Molecules. 26 (12): 3663. doi:10.3390/molecules26123663. PMC 8235047. PMID 34208472.
- ^ Criado-Reyes, Joaquín; Bizzarri, Bruno M.; García-Ruiz, Juan Manuel; Saladino, Raffaele; Di Mauro, Ernesto (October 25, 2021). "The role of borosilicate glass in Miller–Urey experiment". Scientific Reports. 11 (1): 21009. Bibcode:2021NatSR..1121009C. doi:10.1038/s41598-021-00235-4. ISSN 2045-2322. PMC 8545935. PMID 34697338.
- ^ Zahnle, K.; Schaefer, L.; Fegley, B. (October 1, 2010). "Earth's Earliest Atmospheres". Cold Spring Harbor Perspectives in Biology. 2 (10): a004895. doi:10.1101/cshperspect.a004895. ISSN 1943-0264. PMC 2944365. PMID 20573713.
- ^ a b c d e Catling, David C.; Kasting, James F. (2017). Atmospheric Evolution on Inhabited and Lifeless Worlds. Cambridge: Cambridge University Press. doi:10.1017/9781139020558. ISBN 978-0-521-84412-3.
- ^ Rubey, W. W. (1955), "Development of the Hydrosphere and Atmosphere, with Special Reference to Probable Composition of the Early Atmosphere", Geological Society of America Special Papers, vol. 62, pp. 631–650, doi:10.1130/spe62-p631, retrieved November 15, 2023
- ^ Marchi, S.; Bottke, W. F.; Elkins-Tanton, L. T.; Bierhaus, M.; Wuennemann, K.; Morbidelli, A.; Kring, D. A. (2014). "Widespread mixing and burial of Earth's Hadean crust by asteroid impacts". Nature. 511 (7511): 578–582. Bibcode:2014Natur.511..578M. doi:10.1038/nature13539. ISSN 1476-4687. PMID 25079556. S2CID 205239647.
- ^ a b Catling, D., & Kasting, J. (2017). Escape of Atmospheres to Space. In Atmospheric Evolution on Inhabited and Lifeless Worlds (pp. 129–168). Cambridge: Cambridge University Press. doi:10.1017/9781139020558.006
- ^ Tian, Feng; Toon, Owen B.; Pavlov, Alexander A.; De Sterck, H. (May 13, 2005). "A Hydrogen-Rich Early Earth Atmosphere". Science. 308 (5724): 1014–1017. Bibcode:2005Sci...308.1014T. doi:10.1126/science.1106983. ISSN 0036-8075. PMID 15817816. S2CID 262262244.
- ^ Kuramoto, Kiyoshi; Umemoto, Takafumi; Ishiwatari, Masaki (August 1, 2013). "Effective hydrodynamic hydrogen escape from an early Earth atmosphere inferred from high-accuracy numerical simulation". Earth and Planetary Science Letters. 375: 312–318. Bibcode:2013E&PSL.375..312K. doi:10.1016/j.epsl.2013.05.050. ISSN 0012-821X.
- ^ Zahnle, Kevin J.; Gacesa, Marko; Catling, David C. (January 1, 2019). "Strange messenger: A new history of hydrogen on Earth, as told by Xenon". Geochimica et Cosmochimica Acta. 244: 56–85. arXiv:1809.06960. Bibcode:2019GeCoA.244...56Z. doi:10.1016/j.gca.2018.09.017. ISSN 0016-7037.
- ^ Nunn, JF (1998). "Evolution of the atmosphere". Proceedings of the Geologists' Association. Geologists' Association. 109 (1): 1–13. Bibcode:1998PrGA..109....1N. doi:10.1016/s0016-7878(98)80001-1. PMID 11543127.
- ^ Raulin, F; Bossard, A (1984). "Organic syntheses in gas phase and chemical evolution in planetary atmospheres". Advances in Space Research. 4 (12): 75–82. Bibcode:1984AdSpR...4l..75R. doi:10.1016/0273-1177(84)90547-7. PMID 11537798.
- ^ Raulin, François; Brassé, Coralie; Poch, Olivier; Coll, Patrice (2012). "Prebiotic-like chemistry on Titan". Chemical Society Reviews. 41 (16): 5380–93. doi:10.1039/c2cs35014a. PMID 22481630.
- ^ Schmitt-Kopplin, Philippe; Gabelica, Zelimir; Gougeon, Régis D.; Fekete, Agnes; Kanawati, Basem; Harir, Mourad; Gebefuegi, Istvan; Eckel, Gerhard; Hertkorn, Norbert (February 16, 2010). "High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall". Proceedings of the National Academy of Sciences. 107 (7): 2763–2768. Bibcode:2010PNAS..107.2763S. doi:10.1073/pnas.0912157107. ISSN 0027-8424. PMC 2840304. PMID 20160129.
- ^ Shock, Everett L.; Schulte, Mitchell D. (November 1, 1990). "Summary and implications of reported amino acid concentrations in the Murchison meteorite". Geochimica et Cosmochimica Acta. 54 (11): 3159–3173. Bibcode:1990GeCoA..54.3159S. doi:10.1016/0016-7037(90)90131-4. ISSN 0016-7037. PMID 11541223.
- ^ Koga, Toshiki; Naraoka, Hiroshi (April 4, 2017). "A new family of extraterrestrial amino acids in the Murchison meteorite". Scientific Reports. 7 (1): 636. Bibcode:2017NatSR...7..636K. doi:10.1038/s41598-017-00693-9. ISSN 2045-2322. PMC 5428853. PMID 28377577.
- ^ Thompson WR, Murray BG, Khare BN, Sagan C (December 1987). "Coloration and darkening of methane clathrate and other ices by charged particle irradiation: applications to the outer solar system". Journal of Geophysical Research. 92 (A13): 14933–47. Bibcode:1987JGR....9214933T. doi:10.1029/JA092iA13p14933. PMID 11542127.
- ^ PIERAZZO, E.; CHYBA C.F. (2010). "Amino acid survival in large cometary impacts". Meteoritics & Planetary Science. 34 (6): 909–918. Bibcode:1999M&PS...34..909P. doi:10.1111/j.1945-5100.1999.tb01409.x. S2CID 97334519.
- ^ Chyba, Christopher; Sagan, Carl (1992). "Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: an inventory for the origins of life". Nature. 355 (6356): 125–132. Bibcode:1992Natur.355..125C. doi:10.1038/355125a0. ISSN 1476-4687. PMID 11538392. S2CID 4346044.
- ^ a b c Brooks D.J.; Fresco J.R.; Lesk A.M.; Singh M. (October 1, 2002). "Evolution of amino acid frequencies in proteins over deep time: inferred order of introduction of amino acids into the genetic code". Molecular Biology and Evolution. 19 (10): 1645–55. doi:10.1093/oxfordjournals.molbev.a003988. PMID 12270892. Archived from the original on December 13, 2004.
- ^ a b "Why the Miller Urey research argues against abiogenesis". creation.com. Retrieved November 15, 2023.
- ^ Ozturk, S. Furkan; Sasselov, Dimitar D. (July 12, 2022). "On the origins of life's homochirality: Inducing enantiomeric excess with spin-polarized electrons". Proceedings of the National Academy of Sciences. 119 (28): e2204765119. arXiv:2203.16011. Bibcode:2022PNAS..11904765O. doi:10.1073/pnas.2204765119. ISSN 0027-8424. PMC 9282223. PMID 35787048.
- ^ Nelson, D. L., & Cox, M. M. (2017). Lehninger principles of biochemistry (7th ed.). W.H. Freeman.
- ^ Parker, Eric T.; Cleaves, James H.; Burton, Aaron S.; Glavin, Daniel P.; Dworkin, Jason P.; Zhou, Manshui; Bada, Jeffrey L.; Fernández, Facundo M. (January 21, 2014). "Conducting Miller-Urey Experiments". Journal of Visualized Experiments (83): e51039. doi:10.3791/51039. ISSN 1940-087X. PMC 4089479. PMID 24473135.
- ^ Blackmond, Donna G. (2019). "The Origin of Biological Homochirality". Cold Spring Harbor Perspectives in Biology. 11 (3): a032540. doi:10.1101/cshperspect.a032540. ISSN 1943-0264. PMC 6396334. PMID 30824575.
- ^ Ozturk, S. Furkan; Liu, Ziwei; Sutherland, John D.; Sasselov, Dimitar D. (June 9, 2023). "Origin of biological homochirality by crystallization of an RNA precursor on a magnetic surface". Science Advances. 9 (23): eadg8274. arXiv:2303.01394. Bibcode:2023SciA....9G8274O. doi:10.1126/sciadv.adg8274. ISSN 2375-2548. PMC 10246896. PMID 37285423.
- ^ Ozturk, S. Furkan; Bhowmick, Deb Kumar; Kapon, Yael; Sang, Yutao; Kumar, Anil; Paltiel, Yossi; Naaman, Ron; Sasselov, Dimitar D. (October 10, 2023). "Chirality-induced avalanche magnetization of magnetite by an RNA precursor". Nature Communications. 14 (1): 6351. arXiv:2304.09095. Bibcode:2023NatCo..14.6351O. doi:10.1038/s41467-023-42130-8. ISSN 2041-1723. PMC 10564924. PMID 37816811.
- ^ Ozturk, S. Furkan; Sasselov, Dimitar D.; Sutherland, John D. (August 14, 2023). "The central dogma of biological homochirality: How does chiral information propagate in a prebiotic network?". The Journal of Chemical Physics. 159 (6). arXiv:2306.01803. Bibcode:2023JChPh.159f1102O. doi:10.1063/5.0156527. ISSN 0021-9606. PMC 7615580. PMID 37551802.
- ^ Pascal, Robert; Chen, Irene A. (2019). "From soup to peptides". Nature Chemistry. 11 (9): 763–764. Bibcode:2019NatCh..11..763P. doi:10.1038/s41557-019-0318-6. ISSN 1755-4349. PMID 31406322. S2CID 199541746.
- ^ a b Griffith, Elizabeth C.; Vaida, Veronica (September 25, 2012). "In situ observation of peptide bond formation at the water–air interface". Proceedings of the National Academy of Sciences. 109 (39): 15697–15701. Bibcode:2012PNAS..10915697G. doi:10.1073/pnas.1210029109. ISSN 0027-8424. PMC 3465415. PMID 22927374.
- ^ Tirard, S. (2011). Bernal's Conception of Origins of Life. In: Gargaud, M., et al. Encyclopedia of Astrobiology. Springer, Berlin, Heidelberg. [[doi:10.1007/978-3-642-11274-4 158|doi:10.1007/978-3-642-11274-4_158]]
- ^ Erastova V, Degiacomi MT, Fraser D, Greenwell HC (December 2017). "Mineral surface chemistry control for origin of prebiotic peptides". Nature Communications. 8 (1): 2033. Bibcode:2017NatCo...8.2033E. doi:10.1038/s41467-017-02248-y. PMC 5725419. PMID 29229963.
- ^ Canavelli, Pierre; Islam, Saidul; Powner, Matthew W. (2019). "Peptide ligation by chemoselective aminonitrile coupling in water". Nature. 571 (7766): 546–549. doi:10.1038/s41586-019-1371-4. ISSN 1476-4687. PMID 31292542. S2CID 195873596.
External links
edit- A simulation of the Miller–Urey Experiment along with a video Interview with Stanley Miller by Scott Ellis from CalSpace (UCSD)
- Origin-Of-Life Chemistry Revisited: Reanalysis of famous spark-discharge experiments reveals a richer collection of amino acids were formed.
- Miller–Urey experiment explained
- Miller experiment with Lego bricks
- "Stanley Miller's Experiment: Sparking the Building Blocks of Life" on PBS
- The Miller-Urey experiment website
- Cairns-Smith, A.G. (1966). "The origin of life and the nature of the primitive gene". Journal of Theoretical Biology. 10 (1): 53–88. Bibcode:1966JThBi..10...53C. doi:10.1016/0022-5193(66)90178-0. PMID 5964688.
- Details of 2008 re-analysis