Methacholine (INN, USAN) (trade name Provocholine), also known as acetyl-β-methylcholine, is a synthetic choline ester that acts as a non-selective muscarinic receptor agonist in the parasympathetic nervous system.
 | |
| Clinical data | |
|---|---|
| Trade names | Provocholine |
| AHFS/Drugs.com | Consumer Drug Information |
| License data |
|
| Routes of administration | Respiratory |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
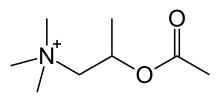
| Formula | C8H18NO2+ |
| Molar mass | 160.237 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Medical uses
editMethacholine is primarily used to diagnose bronchial hyperreactivity,[1] which is the hallmark of asthma and also occurs in chronic obstructive pulmonary disease. This is accomplished through the bronchial challenge test, or methacholine challenge, in which a subject inhales aerosolized methacholine, leading to bronchoconstriction. Other therapeutic uses are limited by its adverse cardiovascular effects, such as bradycardia and hypotension, which arise from its function as a cholinomimetic.
Pharmacology
editIt is highly active at all of the muscarinic receptors, but has little effect on the nicotinic receptors. Methacholine has a charged quaternary amine structure, rendering it insoluble in lipid cell membranes. Clinically, this means that it will not cross the blood–brain barrier and has poor absorption from the gastrointestinal tract. It is broken down at a relatively slow rate within the body, due to its relative resistance to acetylcholinesterases.
The chemical structure of methacholine is identical to acetylcholine but with a methyl group on the beta carbon (hence being called acetyl-β-methylcholine), this β-methyl group provides selectivity toward muscarinic receptors as compared to nicotinic receptors. The quaternary ammonium group is essential for activity. The ester makes it susceptible to the enzyme acetylcholine esterase.[2][unreliable source?]
Contraindications
editUse of methacholine is contraindicated in patients with recent heart attack or stroke, uncontrolled hypertension, known severe airway disease, or aortic aneurysm. It may be used with caution by nursing or pregnant mothers and patients taking certain medications for myasthenia gravis.[3]
References
edit- ^ Birnbaum S, Barreiro TJ (June 2007). "Methacholine challenge testing: identifying its diagnostic role, testing, coding, and reimbursement". Chest. 131 (6): 1932–5. doi:10.1378/chest.06-1385. PMID 17565027.
- ^ "Medicinal Chemistry of the Peripheral Nervous System – Adrenergics and Cholinergic their Biosynthesis, Metabolism and Structure Activity Relationships". pharmaxchange.info. Archived from the original on 2010-11-04. Retrieved 2010-10-16.
- ^ Valentin Popa (2001). "ATS guidelines for methacholine and exercise challenge testing". American Journal of Respiratory and Critical Care Medicine. 163 (1): 292–293. doi:10.1164/ajrccm.163.1.16310b. PMID 11208661.
External links
edit- "Methacholine". Drug Information Portal. U.S. National Library of Medicine.
- "Methacholine chloride". Drug Information Portal. U.S. National Library of Medicine.