A mesoporous material (or super nanoporous [2]) is a nanoporous material containing pores with diameters between 2 and 50 nm, according to IUPAC nomenclature.[3] For comparison, IUPAC defines microporous material as a material having pores smaller than 2 nm in diameter and macroporous material as a material having pores larger than 50 nm in diameter.
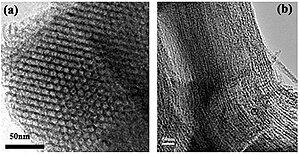
Typical mesoporous materials include some kinds of silica and alumina that have similarly-sized mesopores. Mesoporous oxides of niobium, tantalum, titanium, zirconium, cerium and tin have also been reported. However, the flagship of mesoporous materials is mesoporous carbon, which has direct applications in energy storage devices.[4] Mesoporous carbon has porosity within the mesopore range and this significantly increases the specific surface area. Another very common mesoporous material is activated carbon which is typically composed of a carbon framework with both mesoporosity and microporosity depending on the conditions under which it was synthesized.
According to IUPAC, a mesoporous material can be disordered or ordered in a mesostructure. In crystalline inorganic materials, mesoporous structure noticeably limits the number of lattice units, and this significantly changes the solid-state chemistry. For example, the battery performance of mesoporous electroactive materials is significantly different from that of their bulk structure.[5]
A procedure for producing mesoporous materials (silica) was patented around 1970,[6][7][8] and methods based on the Stöber process from 1968[9] were still in use in 2015.[10] It went almost unnoticed[11] and was reproduced in 1997.[12] Mesoporous silica nanoparticles (MSNs) were independently synthesized in 1990 by researchers in Japan.[13] They were later produced also at Mobil Corporation laboratories[14] and named Mobil Crystalline Materials, or MCM-41.[15] The initial synthetic methods did not allow to control the quality of the secondary level of porosity generated. It was only by employing quaternary ammonium cations and silanization agents during the synthesis that the materials exhibited a true level of hierarchical porosity and enhanced textural properties.[16][17] Mesoporous materials have been also produced in the form of thin films via evaporation induced self-assembly, in different organized mesostructures and compositions.[18]
Since then, research in this field has steadily grown. Notable examples of prospective industrial applications are catalysis, sorption, gas sensing, batteries,[19] ion exchange, optics, and photovoltaics. In the field of catalysis, zeolites is an emerging topic where the mesoporosity as a function of the catalyst is studied to improve its performance for use in Fluid catalytic cracking.
It should be taken into account that this mesoporosity refers to the classification of nanoscale porosity, and mesopores may be defined differently in other contexts; for example, mesopores are defined as cavities with sizes in the range 30 μm–75 μm in the context of porous aggregations such as soil.[20]
See also
editReferences
edit- ^ Guo, M.; Wang, H.; Huang, D.; Han, Z.; Li, Q.; Wang, X.; Chen, J. (2014). "Amperometric catechol biosensor based on laccase immobilized on nitrogen-doped ordered mesoporous carbon (N-OMC)/PVA matrix". Science and Technology of Advanced Materials. 15 (3): 035005. Bibcode:2014STAdM..15c5005G. doi:10.1088/1468-6996/15/3/035005. PMC 5090526. PMID 27877681.
- ^ Mays, T.J. (2007-01-01). "A new classification of pore sizes". Studies in Surface Science and Catalysis. 160: 57–62. doi:10.1016/S0167-2991(07)80009-7. ISBN 9780444520227. ISSN 0167-2991.
- ^ Rouquerol, J.; Avnir, D.; Fairbridge, C. W.; Everett, D. H.; Haynes, J. M.; Pernicone, N.; Ramsay, J. D. F.; Sing, K. S. W.; Unger, K. K. (1994). "Recommendations for the characterization of porous solids (Technical Report)". Pure and Applied Chemistry. 66 (8): 1739–1758. doi:10.1351/pac199466081739. S2CID 18789898.
- ^ Eftekhari, Ali; Zhaoyang, Fan (2017). "Ordered mesoporous carbon and its applications for electrochemical energy storage and conversion". Materials Chemistry Frontiers. 1 (6): 1001–1027. doi:10.1039/C6QM00298F.
- ^ Eftekhari, Ali (2017). "Ordered Mesoporous Materials for Lithium-Ion Batteries". Microporous and Mesoporous Materials. 243: 355–369. Bibcode:2017MicMM.243..355E. doi:10.1016/j.micromeso.2017.02.055.
- ^ Chiola, V.; Ritsko, J. E. and Vanderpool, C. D. "Process for producing low-bulk density silica." Application No. US 3556725D A filed on 26-Feb-1969; Publication No. US 3556725 A published on 19-Jan-1971
- ^ "Porous silica particles containing a crystallized phase and method" Application No. US 3493341D A filed on 23-Jan-1967; Publication No. US 3493341 A published on 03-Feb-1970
- ^ "Process for producing silica in the form of hollow spheres"; Application No. US 342525 A filed on 04-Feb-1964; Publication No. US 3383172 A published on 14-May-1968
- ^ Stöber, Werner; Fink, Arthur; Bohn, Ernst (1968). "Controlled growth of monodisperse silica spheres in the micron size range". Journal of Colloid and Interface Science. 26 (1): 62–69. Bibcode:1968JCIS...26...62S. doi:10.1016/0021-9797(68)90272-5.
- ^ Kicklebick, Guido (2015). "Nanoparticles and Composites". In Levy, David; Zayat, Marcos (eds.). The Sol-Gel Handbook: Synthesis, Characterization and Applications. Vol. 3. John Wiley & Sons. pp. 227–244. ISBN 9783527334865.
- ^ Xu, Ruren; Pang, Wenqin & Yu, Jihong (2007). Chemistry of zeolites and related porous materials: synthesis and structure. Wiley-Interscience. p. 472. ISBN 978-0-470-82233-3.
- ^ Direnzo, F; Cambon, H; Dutartre, R (1997). "A 28-year-old synthesis of micelle-templated mesoporous silica". Microporous Materials. 10 (4–6): 283. doi:10.1016/S0927-6513(97)00028-X.
- ^ Yanagisawa, Tsuneo; Shimizu, Toshio; Kuroda, Kazuyuki; Kato, Chuzo (1990). "The preparation of alkyltrimethylammonium-kanemite complexes and their conversion to microporous materials". Bulletin of the Chemical Society of Japan. 63 (4): 988. doi:10.1246/bcsj.63.988.
- ^ Beck, J. S.; Vartuli, J. C.; Roth, W. J.; Leonowicz, M. E.; Kresge, C. T.; Schmitt, K. D.; Chu, C. T. W.; Olson, D. H.; Sheppard, E. W. (1992). "A new family of mesoporous molecular sieves prepared with liquid crystal templates". Journal of the American Chemical Society. 114 (27): 10834. doi:10.1021/ja00053a020.
- ^ Trewyn, B. G.; Slowing, I. I.; Giri, S.; Chen, H. T.; Lin, V. S. -Y. (2007). "Synthesis and Functionalization of a Mesoporous Silica Nanoparticle Based on the Sol–Gel Process and Applications in Controlled Release". Accounts of Chemical Research. 40 (9): 846–53. doi:10.1021/ar600032u. PMID 17645305.
- ^ Perez-Ramirez, J.; Christensen, C. H.; Egeblad, K.; Christensen, C. H.; Groen, J. C. (2008). "Hierarchical zeolites: enhanced utilisation of microporous crystals in catalysis by advances in materials design". Chem. Soc. Rev. 37 (11): 2530–2542. doi:10.1039/b809030k. PMID 18949124.
- ^ Perez-Ramirez, J.; Verboekend, D. (2011). "Design of hierarchical zeolite catalysts by desilication". Catal. Sci. Technol. 1 (6): 879–890. doi:10.1039/C1CY00150G. hdl:20.500.11850/212833.
- ^ Innocenzi, Plinio (2022). Mesoporous ordered silica films. From self-assembly to order. Springer. ISBN 978-3-030-89535-8.
- ^ Stein, Andreas (2020). Gitis, Vitaly; Rothenberg, Gadi (eds.). Handbook of Porous Materials. Vol. 4. Singapore: WORLD SCIENTIFIC. doi:10.1142/11909. ISBN 978-981-12-2322-8.
- ^ Soil Science Glossary Terms Committee (2008). Glossary of Soil Science Terms 2008. Madison, WI: Soil Science Society of America. ISBN 978-0-89118-851-3.