In organic chemistry, the Le Bel–Van 't Hoff rule states that the number of stereoisomers of an organic compound containing no internal planes of symmetry is 2n, where n represents the number of asymmetric carbon atoms. French chemist Joseph Achille Le Bel[1] and Dutch chemist Jacobus Henricus van 't Hoff[2] both announced this hypothesis in 1874 and that this accounted for all molecular asymmetry known at the time.[3]
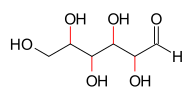
As an example, four of the carbon atoms of the aldohexose class of molecules are asymmetric, therefore the Le Bel–Van 't Hoff rule gives a calculation of 24 = 16 stereoisomers. This is indeed the case: these chemicals are two enantiomers each of eight different diastereomers: allose, altrose, glucose, mannose, gulose, idose, galactose, and talose.
References
edit- ^ Le Bel, Joseph Achille (1874). "Sur les relations qui existent entre les formules atomiques des corps organiques et le pouvoir rotatoire de leurs dissolutions" [On the relations that exist between the atomic formulas of organic substances and the rotatory power of their solutions]. Bulletin de la Société Chimique de Paris (in French). 22: 337–347.
- ^ Van 't Hoff, Jacobus Henricus (1874). "Sur les formules de structure dans l'espace" [On structural formulas in space]. Archives Néerlandaises des Sciences Exactes et Naturelles (in French). 9: 445–454.
- ^ "Le Bel–van 't Hoff rule". TheFreeDictionary's Medical dictionary.