Omacetaxine mepesuccinate (INN; trade name Synribo; formerly named as homoharringtonine or HHT) is a pharmaceutical drug substance that is indicated for treatment of chronic myeloid leukemia (CML).
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Synribo |
| AHFS/Drugs.com | Monograph |
| License data | |
| Routes of administration | Subcutaneous, intravenous infusion |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 50% |
| Metabolism | Mostly via plasma esterases |
| Elimination half-life | 6 hours |
| Excretion | Urine (≤15% unchanged) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.164.439 |
| Chemical and physical data | |
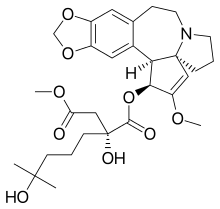
| Formula | C29H39NO9 |
| Molar mass | 545.629 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
HHT is a natural plant alkaloid derived from Cephalotaxus fortunei. HHT and related compound esters of cephalotaxine were described first in 1970, and were the subject of intensive research efforts by Chinese investigators to clarify their role as anticancer and antileukemic agents from the 1970s until the present.[1] It was approved by the US FDA in October 2012 for the treatment of adult patients with CML with resistance and/or intolerance to two or more tyrosine kinase inhibitors (TKIs).[2]
Medical uses
editOmacetaxine/homoharringtonine is indicated for use as a treatment for patients with chronic myeloid leukaemia who are resistant or intolerant of tyrosine kinase inhibitors.[3][4][5]
In June 2009, results of a long-term open label Phase II study were published, which investigated the use of omacetaxine infusions in CML patients. After twelve months of treatment, about one third of patients showed a cytogenetic response.[6] A study in patients who had failed imatinib and who had the drug resistant T315I mutation achieved cytogenetic response in 28% of patients and hematologic response in 80% of patients, according to preliminary data.[7]
Phase I studies including a small number of patients have shown benefit in treating myelodysplastic syndrome (MDS, 25 patients)[8] and acute myelogenous leukaemia (AML, 76 patients).[9] Patients with solid tumors did not benefit from omacetaxine.[10]
Adverse effects
editBy frequency:[2][3]
Very common (>10% frequency):
- Diarrhea
- Myelosuppression†
- Injection site reactions
- Nausea
- Fatigue
- Fever
- Muscle weakness
- Joint pain
- Headache
- Cough
- Hair loss
- Constipation
- Nosebleeds
- Upper abdominal pain
- Pain in the extremities
- Oedema
- Vomiting
- Back pain
- Hyperglycemia, sometimes extreme
- Gout
- Rash
- Insomnia
Common (1–10% frequency):
- Seizures
- Haemorrhage
† Myelosuppression, including: thrombocytopenia, anaemia, neutropenia and lymphopenia, in descending order of frequency.
Omacetaxine mepesuccinate can cause fetal harm when administered to a pregnant woman. Women using HHT should avoid becoming pregnant and also avoid nursing while receiving HHT.[11]
Mechanism of action
editOmacetaxine mepesuccinate is a protein translation inhibitor. It inhibits protein translation by preventing the initial elongation step of protein synthesis. It interacts with the ribosomal A-site and prevents the correct positioning of amino acid side chains of incoming aminoacyl-tRNAs. Omacetaxine mepesuccinate acts only on the initial step of protein translation and does not inhibit protein synthesis from mRNAs that have already commenced translation.[12]
References
edit- ^ Kantarjian HM, O'Brien S, Cortes J (October 2013). "Homoharringtonine/omacetaxine mepesuccinate: the long and winding road to food and drug administration approval". Clinical Lymphoma, Myeloma & Leukemia. 13 (5): 530–3. doi:10.1016/j.clml.2013.03.017. PMC 3775965. PMID 23790799.
- ^ a b "Synribo (omacetaxine) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 18 February 2014.
- ^ a b "SYNRIBO (omacetaxine mepesuccinate) injection, powder, lyophilized, for solution [Cephalon, Inc.]". DailyMed. Cephalon, Inc. October 2012. Retrieved 18 February 2014.
- ^ Sweetman, S, ed. (14 November 2012). "Omacetaxine Mepesuccinate". Martindale: The Complete Drug Reference. Medicines Complete. Pharmaceutical Press.
- ^ Lacroix M (2014). Targeted Therapies in Cancer. Hauppauge , NY: Nova Sciences Publishers. ISBN 978-1-63321-687-7. Archived from the original on 2015-06-26. Retrieved 2014-07-13.
- ^ Li YF, Deng ZK, Xuan HB, Zhu JB, Ding BH, Liu XN, Chen BA (June 2009). "Prolonged chronic phase in chronic myelogenous leukemia after homoharringtonine therapy". Chinese Medical Journal. 122 (12): 1413–7. PMID 19567163.
- ^ Quintás-Cardama A, Kantarjian H, Cortes J (December 2009). "Homoharringtonine, omacetaxine mepesuccinate, and chronic myeloid leukemia circa 2009". Cancer. 115 (23): 5382–93. doi:10.1002/cncr.24601. PMID 19739234.
- ^ Wu L, Li X, Su J, Chang C, He Q, Zhang X, et al. (September 2009). "Effect of low-dose cytarabine, homoharringtonine and granulocyte colony-stimulating factor priming regimen on patients with advanced myelodysplastic syndrome or acute myeloid leukemia transformed from myelodysplastic syndrome". Leukemia & Lymphoma. 50 (9): 1461–7. doi:10.1080/10428190903096719. PMID 19672772. S2CID 10675425.
- ^ Gu LF, Zhang WG, Wang FX, Cao XM, Chen YX, He AL, et al. (June 2011). "Low dose of homoharringtonine and cytarabine combined with granulocyte colony-stimulating factor priming on the outcome of relapsed or refractory acute myeloid leukemia". Journal of Cancer Research and Clinical Oncology. 137 (6): 997–1003. doi:10.1007/s00432-010-0947-z. PMID 21152934. S2CID 20406046.
- ^ Kantarjian HM, Talpaz M, Santini V, Murgo A, Cheson B, O'Brien SM (September 2001). "Homoharringtonine: history, current research, and future direction". Cancer. 92 (6): 1591–605. doi:10.1002/1097-0142(20010915)92:6<1591::AID-CNCR1485>3.0.CO;2-U. PMID 11745238. S2CID 221577386.
- ^ "SYNRIBOTM (omacetaxine mepesuccinate) drug label" (PDF). FDA.
- ^ Wetzler M, Segal D (2011). "Omacetaxine as an anticancer therapeutic: what is old is new again". Current Pharmaceutical Design. 17 (1): 59–64. doi:10.2174/138161211795049778. PMID 21294709.