Pentosan polysulfate, sold under the brand name Elmiron among others, is a medication used for the treatment of interstitial cystitis.[1] It was approved for medical use in the United States in 1996.[1][2][3]
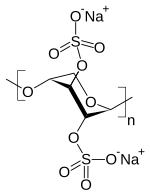 | |
| Clinical data | |
|---|---|
| Trade names | Elmiron, Zycosan |
| Other names | PPS, (1->4)-β-Xylan 2,3-bis(hydrogen sulfate) with a 4 O-methyl-α-D-glucuronate |
| AHFS/Drugs.com | Monograph |
| License data | |
| Routes of administration | By mouth, intramuscular |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Excretion | Feces, urine[1] |
| Identifiers | |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C10H18O21S4 |
| Molar mass | 602.47 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Medical uses
editPentosan polysulfate sodium is indicated for the relief of bladder pain or discomfort associated with interstitial cystitis.[1]
Adverse effects
editPatients who have taken pentosan polysulfate orally report a variety of side effects, primarily gastrointestinal complaints such as diarrhea, heartburn, and stomach pain.[4] Hair loss, headache, rash, and insomnia have also been reported.[4] Due to Elmiron's anticoagulant effects, some patients report bruising more easily. In some cases, patients are asked to stop taking the medication before any major surgical procedures to reduce the likelihood of bleeding. Recent report based on clinical observation hypothesizes that chronic exposure to pentosan polysulfate can cause retinal toxicity, mimicking pigmentary pattern dystrophy.[2][5][6][7]
Mechanism of action
editIn interstitial cystitis, pentosan polysulfate is believed to work by providing a protective coating to the damaged bladder wall. Pentosan polysulfate is similar in structure to the natural glycosaminoglycan coating of the inner lining of the bladder, and may replace or repair the lining, reducing its permeability.[5]
History
editThe calcium salt of pentosan polysulfate was one of the first reported disease-modifying osteoarthritis drugs (DMOAD).[6]
Society and culture
editNames
editThe IUPAC name for pentosan polysulfate is [(2R,3R,4S,5R)-2-hydroxy-5-[(2S,3R,4S,5R)-5-hydroxy-3,4-disulfooxyoxan-2-yl]oxy-3-sulfooxyoxan-4-yl] hydrogen sulfate.[8]
There are 40 synonyms listed for pentosan polysulfate on PubChem including BAY-946, HOE-946, pentosan sulfuric polyester, polypentose sulfate, polysulfated xylan, PZ-68, SP-54, xylan SP54 and xylan sulfate.[9]
Various brand names include Elmiron (as sodium salt), Hemoclar, Anarthron, Fibrase, Fibrocid, Thrombocid and SP54. Pentosan polysulfate capsules are sold in India under the brand names Comfora, Pentossan-100, Cystopen and For-IC. In the veterinary field, pentosan polysulfate is sold as Cartrophen Vet and Sylvet by Biopharm Australia, Pentosan by Naturevet Australia, Anarthron by Randlab Australia and Zydax by Parnell.[10]
Research
editOsteoarthritis
editPentosan polysulfate has been studied in knee osteoarthritis, though evidence to support such use is poor as of 2003.[11] There is some theoretical evidence that it should help.[12]
Transmissible spongiform encephalopathies
editPentosan polysulfate is being studied as a potential treatment of Creutzfeldt–Jakob disease (CJD). The rationale for this treatment was unclear but it was subsequently shown in prion-infected mouse neuroblastoma cells that pentosan polysulfate could rapidly reduce the levels of abnormal (scrapie) prion without affecting the normal cellular isoform.[13] As pentosan polysulfate can bind to the cellular isoform of the prion protein, it may stabilise this form and prevent its conversion to the pathological (scrapie) isoform.[14]
The treatment[15] of one patient in Northern Ireland and around six other patients in mainland Britain was reported in the press.[16]
Veterinary uses
editDogs
editRead et al. (1996) [17] used three different doses of sodium pentosan polysulfate to treat 40 geriatric dogs with well-established clinical signs of chronic osteoarthritis (OA) with a subcutaneous injection. The 3 mg/kg dose was the most effective. In a study conducted with 10 elderly dogs with osteoarthritis given calcium pentosan polysulfate (3 mg/kg intramuscularly) once weekly for four weeks, the improvement in symptoms (seen at 1, 2, 3 and 7 weeks after initiation of therapy) was found to correlate with plasma indices of fibrinolytic activity and lipid profiles.[18] In a study in dogs with OA secondary to cranial cruciate ligament deficiency, although no differences were identified in either functional outcome or radiographic progression using the oral calcium pentosan polysulfate compared with placebo, there were significantly lower levels of proteoglycan breakdown products in the synovial fluid of the osteoarthritic joints.[19] The efficacy of subcutaneous sodium pentosan polysulfate (3 mg/kg) was tested in 40 dogs with cranial cruciate ligament instability and found to hasten recovery, as measured by more rapidly improved ground reaction forces, over 48 weeks.[20]
Horses
editZycosan, for horses, is a heparin-like compound and is the first injectable pentosan product to receive approval by the US Food and Drug Administration (FDA).[21]
In December 2022, the US Food and Drug Administration (FDA) approved pentosan polysulfate (Zycosan) for the control of clinical signs associated with osteoarthritis in horses.[21] Zycosan is for intramuscular use in horses only and is not for use in humans.[21] Zycosan is sponsored by Anzac Animal Health LLC, based in Maryland Heights, Missouri.[21]
Pentosan polysulfate is being used for this osteoarthritis in Australia. When administered to racing thoroughbreds with chronic osteoarthritis (2 to 3 mg/kg, intramuscularly, once weekly for 4 weeks, then as required), pentosan polysulfate treatment improved but did not eliminate clinical signs of joint disease.[22] Articular cartilage fibrillation was substantially reduced by similar NaPPS treatment intramuscularly in nine horses with experimentally-induced carpal osteoarthritis.[23]
References
edit- ^ a b c d e "Elmiron- pentosan polysulfate sodium capsule, gelatin coated". DailyMed. 10 November 2022. Retrieved 21 December 2022.
- ^ a b Lindeke-Myers A, Hanif AM, Jain N (2022). "Pentosan polysulfate maculopathy". Survey of Ophthalmology. 67 (1): 83–96. doi:10.1016/j.survophthal.2021.05.005. PMID 34000253. S2CID 234767956.
- ^ "Elmiron (pentosan polysulfate sodium)" (PDF). FDA approval letter. U.S. Food and Drug Administration. 25 September 1996.
- ^ a b Pubmed Health (2012). "Pentosan Polysulfate". U.S. National Library of Medicine. Retrieved 2 October 2012.
- ^ a b Parsons CL (February 1994). "The therapeutic role of sulfated polysaccharides in the urinary bladder". The Urologic Clinics of North America. 21 (1): 93–100. doi:10.1016/S0094-0143(21)00597-8. PMID 7904388.
- ^ a b Ghosh P, Smith M (2002). "Osteoarthritis, genetic and molecular mechanisms". Biogerontology. 3 (1–2): 85–88. doi:10.1023/a:1015219716583. PMID 12014849. S2CID 33755966.
- ^ Hanif AM, Armenti ST, Taylor SC, Shah RA, Igelman AD, Jayasundera KT, et al. (November 2019). "Phenotypic Spectrum of Pentosan Polysulfate Sodium-Associated Maculopathy: A Multicenter Study". JAMA Ophthalmology. 137 (11): 1275–1282. doi:10.1001/jamaophthalmol.2019.3392. PMC 6735406. PMID 31486843.
- ^ "Pentosan polysulfate". PubChem. U.S. National Library of Medicine. Retrieved 8 November 2022.
- ^ "Pentosan polysulfate". PubChem. U.S. National Library of Medicine. Retrieved 8 November 2022.
- ^ Hannon RL, Smith JG, Cullis-Hill D, Ghosh P, Cawdery MJ (May 2003). "Safety of Cartrophen Vet in the dog: review of adverse reaction reports in the UK". The Journal of Small Animal Practice. 44 (5): 202–208. doi:10.1111/j.1748-5827.2003.tb00144.x. PMID 12779171.
- ^ Uthman I, Raynauld JP, Haraoui B (August 2003). "Intra-articular therapy in osteoarthritis". Postgraduate Medical Journal. 79 (934): 449–453. doi:10.1136/pmj.79.934.449. PMC 1742771. PMID 12954956.
- ^ Ghosh P (February 1999). "The pathobiology of osteoarthritis and the rationale for the use of pentosan polysulfate for its treatment". Seminars in Arthritis and Rheumatism. 28 (4): 211–267. doi:10.1016/s0049-0172(99)80021-3. PMID 10073500.
- ^ Yamasaki T, Suzuki A, Hasebe R, Horiuchi M (2014). "Comparison of the anti-prion mechanism of four different anti-prion compounds, anti-PrP monoclonal antibody 44B1, pentosan polysulfate, chlorpromazine, and U18666A, in prion-infected mouse neuroblastoma cells". PLOS ONE. 9 (9): e106516. Bibcode:2014PLoSO...9j6516Y. doi:10.1371/journal.pone.0106516. PMC 4152300. PMID 25181483.
- ^ Kamatari YO, Hayano Y, Yamaguchi K, Hosokawa-Muto J, Kuwata K (January 2013). "Characterizing antiprion compounds based on their binding properties to prion proteins: implications as medical chaperones". Protein Science. 22 (1): 22–34. doi:10.1002/pro.2180. PMC 3575857. PMID 23081827.
- ^ Whittle IR, Knight RS, Will RG (June 2006). "Unsuccessful intraventricular pentosan polysulphate treatment of variant Creutzfeldt-Jakob disease". Acta Neurochirurgica. 148 (6): 677–9, discussion 679. doi:10.1007/s00701-006-0772-y. PMID 16598408. S2CID 37400744.
- ^ "Research will now assess CJD drug". 1 March 2005 – via news.bbc.co.uk.
- ^ Read RA, Cullis-Hill D, Jones MP (March 1996). "Systemic use of pentosan polysulphate in the treatment of osteoarthritis". The Journal of Small Animal Practice. 37 (3): 108–114. doi:10.1111/j.1748-5827.1996.tb02355.x. PMID 8683953.
- ^ Ghosh P, Cheras PA (December 2001). "Vascular mechanisms in osteoarthritis". Best Practice & Research. Clinical Rheumatology. 15 (5): 693–709. doi:10.1053/berh.2001.0188. PMID 11812016.
- ^ Innes JF, Barr AR, Sharif M (April 2000). "Efficacy of oral calcium pentosan polysulphate for the treatment of osteoarthritis of the canine stifle joint secondary to cranial cruciate ligament deficiency". The Veterinary Record. 146 (15): 433–437. doi:10.1136/vr.146.15.433. PMID 10811265. S2CID 46623547.
- ^ Budsberg SC, Bergh MS, Reynolds LR, Streppa HK (April 2007). "Evaluation of pentosan polysulfate sodium in the postoperative recovery from cranial cruciate injury in dogs: a randomized, placebo-controlled clinical trial". Veterinary Surgery. 36 (3): 234–244. doi:10.1111/j.1532-950x.2007.00256.x. PMID 17461948.
- ^ a b c d "FDA Approves First Injectable Pentosan for Osteoarthritis in Horses". U.S. Food and Drug Administration (FDA). 20 December 2022. Retrieved 21 December 2022. This article incorporates text from this source, which is in the public domain.
- ^ Little CB, Ghosh P (1996). McIlwraith CW, Trotter GW (eds.). Joint Disease in the Horse. Philadelphia: WB Saunders Company. pp. 281–292.
- ^ McIlwraith CW, Frisbie DD, Kawcak CE (May 2012). "Evaluation of intramuscularly administered sodium pentosan polysulfate for treatment of experimentally induced osteoarthritis in horses". American Journal of Veterinary Research. 73 (5): 628–633. doi:10.2460/ajvr.73.5.628. PMID 22533393.