Cytochrome P450 reductase (also known as NADPH:ferrihemoprotein oxidoreductase, NADPH:hemoprotein oxidoreductase, NADPH:P450 oxidoreductase, P450 reductase, POR, CPR, CYPOR) is a membrane-bound enzyme required for electron transfer from NADPH to cytochrome P450[5] and other heme proteins including heme oxygenase in the endoplasmic reticulum[6] of the eukaryotic cell.
Gene
editHuman POR gene has 16 exons and the exons 2-16 code for a 677-amino acid [7] POR protein (NCBI NP_000932.2). There is a single copy of 50 kb POR gene (NCBI NM_000941.2) in humans on chromosome 7 (7q11.23).
Paralogs of POR include nitric oxide synthase (EC 1.14.13.39), NADPH:sulfite reductase (EC 1.8.1.2), and methionine synthase reductase (EC 1.16.1.8).[citation needed]
Protein structure
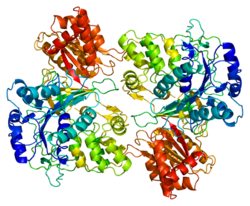
editThe 3D crystal structure of human POR has been determined.[8] The molecule is composed of four structural domains: the FMN-binding domain, the connecting domain, the FAD-binding domain, and NADPH-binding domain. The FMN-binding domain is similar to the structure of FMN-containing protein flavodoxin, whereas the FAD-binding domain and NADPH-binding domains are similar to those of flavoprotein ferredoxin-NADP+ reductase (FNR). The connecting domain is situated between the flavodoxin-like and FNR-like domains. Conformation flexibility of POR is a key requirement for interaction with different redox partners like Cytochrome P450 proteins, and biasing the conformation of POR with small molecule ligands may be a way to control interaction with partner proteins and influence metabolism.[9]
Function
editIn Bacillus megaterium and Bacillus subtilis, POR is a C-terminal domain of CYP102, a single-polypeptide self-sufficient soluble P450 system (P450 is an N-terminal domain). The general scheme of electron flow in the POR/P450 system is:
The definitive evidence for the requirement of POR in cytochrome-P450-mediated reactions came from the work of Lu, Junk and Coon,[10] who dissected the P450-containing mixed function oxidase system into three constituent components: POR, cytochrome P450, and lipids.
Since all microsomal P450 enzymes require POR for catalysis, it is expected that disruption of POR would have devastating consequences. POR knockout mice are embryonic lethal,[11] probably due to lack of electron transport to extrahepatic P450 enzymes since liver-specific knockout of POR yields phenotypically and reproductively normal mice that accumulate hepatic lipids and have remarkably diminished capacity of hepatic drug metabolism.[12]
The reduction of cytochrome P450 is not the only physiological function of POR. The final step of heme oxidation by mammalian heme oxygenase requires POR and O2. In yeast, POR affects the ferrireductase activity, probably transferring electrons to the flavocytochrome ferric reductase.[13]
Clinical significance
editMore than 200 variations in POR gene have been identified.[14][15]
Five missense mutations (A287P, R457H, V492E, C569Y, and V608F) and a splicing mutation in the POR genes have been found in patients who had hormonal evidence for combined deficiencies of two steroidogenic cytochrome P450 enzymes - P450c17 CYP17A1, which catalyzes steroid 17α-hydroxylation and 17,20 lyase reaction, and P450c21 21-hydroxylase, which catalyzes steroid 21-hydroxylation.[16] Another POR missense mutation Y181D has also been identified.[17] Fifteen of nineteen patients having abnormal genitalia and disordered steroidogenesis were homozygous or apparent compound heterozygous for POR mutations that destroyed or dramatically inhibited POR activity.[18]
POR Deficiency – Mixed Oxidase Disease
editPOR deficiency is the newest form of congenital adrenal hyperplasia first described in 2004.[16] The index patient was a newborn 46,XX Japanese girl with craniosynostosis, hypertelorism, mid-face hypoplasia, radiohumeral synostosis, arachnodactyly and disordered steroidogenesis. However, the clinical and biochemical characteristics of patients with POR deficiency are long known in the literature as so-called mixed oxidase disease, as POR deficiency typically shows a steroid profile that suggests combined deficiencies of steroid 21-hydroxylase and 17α-hydroxylase/17,20 lyase activities. The clinical spectrum of POR deficiency ranges from severely affected children with ambiguous genitalia, adrenal insufficiency, and the Antley-Bixler skeletal malformation syndrome (ABS) to mildly affected individuals with polycystic ovary syndrome-like features. Some of the POR patients were born to mothers who became virilized during pregnancy, suggesting deficient placental aromatization of fetal androgens due to a lesion in microsomal aromatase resulting in low estrogen production, which was later confirmed by lower aromatase activities caused by POR mutations.[19][20] However, it has also been suggested that fetal and maternal virilization in POR deficiency might be caused by increased dihydrotestosterone synthesis by the fetal gonad through an alternative "backdoor pathway" first described in the marsupials and later confirmed in humans.[21] Gas chromatography/mass spectrometry analysis of urinary steroids from pregnant women carrying a POR-deficient fetus described in an earlier report also supports the existence of this pathway,[22][23] and the relevance of the backdoor pathway along with POR dependent steroidogenesis have become clearer from recent studies.[21] The role of POR mutations beyond CAH are being investigated; and questions such as how POR mutations cause bony abnormalities and what role POR variants play in drug metabolism by hepatic P450s are being addressed in recent publications.[24][25][26][27][28] However, reports of ABS in some offspring of mothers who were treated with fluconazole, an antifungal agent which interferes with cholesterol biosynthesis at the level of CYP51 activity - indicate that disordered drug metabolism may result from deficient POR activity.[29]
Williams syndrome
editWilliams syndrome is a genetic disorder characterized by the deletion of genetic material approximately 1.2 Mb from the POR gene (POR). Cells with this genetic deletion show reduced transcription of POR, it seems, due to the loss of a cis-regulatory element that alters expression of this gene.[30] Some persons with Williams syndrome show characteristics of POR deficiency, including radioulnar synostosis and other skeletal abnormalities.[31] Cases of mild impairment of cortisol and androgen synthesis have been noted,[32] however, despite the fact that deficient POR impairs androgen synthesis, patients with Williams syndrome often show increased androgen levels.[33] A similar increase in testosterone has been observed in a mouse model that has globally decreased POR expression.[34]
See also
editReferences
edit- ^ a b c GRCh38: Ensembl release 89: ENSG00000127948 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000005514 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Pandey AV, Flück CE (May 2013). "NADPH P450 oxidoreductase: structure, function, and pathology of diseases". Pharmacology & Therapeutics. 138 (2): 229–54. doi:10.1016/j.pharmthera.2013.01.010. PMID 23353702.
- ^ Jensen K, Møller BL (February 2010). "Plant NADPH-cytochrome P450 oxidoreductases". Phytochemistry. 71 (2–3): 132–41. Bibcode:2010PChem..71..132J. doi:10.1016/j.phytochem.2009.10.017. PMID 19931102.
CPR was shown to be localized in the endoplasmic reticulum in the early 1960s (Williams and Kamin, 1962).
- ^ Haniu M, McManus ME, Birkett DJ, Lee TD, Shively JE (October 1989). "Structural and functional analysis of NADPH-cytochrome P-450 reductase from human liver: complete sequence of human enzyme and NADPH-binding sites". Biochemistry. 28 (21): 8639–45. doi:10.1021/bi00447a054. PMID 2513880.
- ^ PDB: 3QE2); Xia C, Panda SP, Marohnic CC, Martásek P, Masters BS, Kim JJ (August 2011). "Structural basis for human NADPH-cytochrome P450 oxidoreductase deficiency". Proceedings of the National Academy of Sciences of the United States of America. 108 (33): 13486–91. Bibcode:2011PNAS..10813486X. doi:10.1073/pnas.1106632108. PMC 3158178. PMID 21808038.
- ^ Jensen SB, Thodberg S, Parween S, Moses ME, Hansen CC, Thomsen J, et al. (2021-04-15). "Biased cytochrome P450-mediated metabolism via small-molecule ligands binding P450 oxidoreductase". Nature Communications. 12 (1): 2260. Bibcode:2021NatCo..12.2260J. doi:10.1038/s41467-021-22562-w. PMC 8050233. PMID 33859207.
- ^ Lu AY, Junk KW, Coon MJ (July 1969). "Resolution of the cytochrome P-450-containing omega-hydroxylation system of liver microsomes into three components". The Journal of Biological Chemistry. 244 (13): 3714–21. doi:10.1016/S0021-9258(18)83427-5. PMID 4389465.
- ^ Shen AL, O'Leary KA, Kasper CB (February 2002). "Association of multiple developmental defects and embryonic lethality with loss of microsomal NADPH-cytochrome P450 oxidoreductase". The Journal of Biological Chemistry. 277 (8): 6536–41. doi:10.1074/jbc.M111408200. PMID 11742006.
- ^ Gu J, Weng Y, Zhang QY, Cui H, Behr M, Wu L, Yang W, Zhang L, Ding X (July 2003). "Liver-specific deletion of the NADPH-cytochrome P450 reductase gene: impact on plasma cholesterol homeostasis and the function and regulation of microsomal cytochrome P450 and heme oxygenase". The Journal of Biological Chemistry. 278 (28): 25895–901. doi:10.1074/jbc.M303125200. PMID 12697746.
- ^ Lesuisse E, Casteras-Simon M, Labbe P (November 1997). "Cytochrome P-450 reductase is responsible for the ferrireductase activity associated with isolated plasma membranes of Saccharomyces cerevisiae". FEMS Microbiology Letters. 156 (1): 147–52. doi:10.1111/j.1574-6968.1997.tb12720.x. PMID 9368374.
- ^ Pandey AV, Sproll P (214). "Pharmacogenomics of human P450 oxidoreductase". Frontiers in Pharmacology. 5: 103. doi:10.3389/fphar.2014.00103. PMC 4023047. PMID 24847272.
- ^ Burkhard FZ, Parween S, Udhane SS, Flück CE, Pandey AV (April 2016). "P450 Oxidoreductase deficiency: Analysis of mutations and polymorphisms". The Journal of Steroid Biochemistry and Molecular Biology. 165 (Pt A): 38–50. doi:10.1016/j.jsbmb.2016.04.003. PMID 27068427.
- ^ a b Flück CE, Tajima T, Pandey AV, Arlt W, Okuhara K, Verge CF, Jabs EW, Mendonça BB, Fujieda K, Miller WL (March 2004). "Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome". Nature Genetics. 36 (3): 228–30. doi:10.1038/ng1300. PMID 14758361.
- ^ Arlt W, Walker EA, Draper N, Ivison HE, Ride JP, Hammer F, Chalder SM, Borucka-Mankiewicz M, Hauffa BP, Malunowicz EM, Stewart PM, Shackleton CH (June 2004). "Congenital adrenal hyperplasia caused by mutant P450 oxidoreductase and human androgen synthesis: analytical study". Lancet. 363 (9427): 2128–35. doi:10.1016/S0140-6736(04)16503-3. PMID 15220035. S2CID 32705841.
- ^ Huang N, Pandey AV, Agrawal V, Reardon W, Lapunzina PD, Mowat D, Jabs EW, Van Vliet G, Sack J, Flück CE, Miller WL (May 2005). "Diversity and function of mutations in p450 oxidoreductase in patients with Antley-Bixler syndrome and disordered steroidogenesis". American Journal of Human Genetics. 76 (5): 729–49. doi:10.1086/429417. PMC 1199364. PMID 15793702.
- ^ Parween S, Fernández-Cancio M, Benito-Sanz S, Camats N, Rojas Velazquez MN, López-Siguero JP, et al. (April 2020). "Molecular Basis of CYP19A1 Deficiency in a 46,XX Patient With R550W Mutation in POR: Expanding the PORD Phenotype". The Journal of Clinical Endocrinology and Metabolism. 105 (4): e1272–e1290. doi:10.1210/clinem/dgaa076. PMID 32060549.
- ^ Pandey AV, Kempná P, Hofer G, Mullis PE, Flück CE (October 2007). "Modulation of human CYP19A1 activity by mutant NADPH P450 oxidoreductase". Molecular Endocrinology. 21 (10): 2579–95. doi:10.1210/me.2007-0245. PMID 17595315.
- ^ a b Flück CE, Meyer-Böni M, Pandey AV, Kempná P, Miller WL, Schoenle EJ, Biason-Lauber A (August 2011). "Why boys will be boys: two pathways of fetal testicular androgen biosynthesis are needed for male sexual differentiation". American Journal of Human Genetics. 89 (2): 201–18. doi:10.1016/j.ajhg.2011.06.009. PMC 3155178. PMID 21802064.
- ^ Reisch N, Taylor AE, Nogueira EF, Asby DJ, Dhir V, Berry A, Krone N, Auchus RJ, Shackleton CH, Hanley NA, Arlt W (October 2019). "Alternative pathway androgen biosynthesis and human fetal female virilization". Proceedings of the National Academy of Sciences of the United States of America. 116 (44): 22294–22299. Bibcode:2019PNAS..11622294R. doi:10.1073/pnas.1906623116. PMC 6825302. PMID 31611378.
- ^ Shackleton C, Marcos J, Arlt W, Hauffa BP (August 2004). "Prenatal diagnosis of P450 oxidoreductase deficiency (ORD): a disorder causing low pregnancy estriol, maternal and fetal virilization, and the Antley-Bixler syndrome phenotype". American Journal of Medical Genetics Part A. 129A (2): 105–12. doi:10.1002/ajmg.a.30171. PMID 15316970. S2CID 22583190.
- ^ Flück CE, Mullis PE, Pandey AV (October 2010). "Reduction in hepatic drug metabolizing CYP3A4 activities caused by P450 oxidoreductase mutations identified in patients with disordered steroid metabolism". Biochemical and Biophysical Research Communications. 401 (1): 149–53. doi:10.1016/j.bbrc.2010.09.035. PMID 20849814.
- ^ Tomalik-Scharte D, Maiter D, Kirchheiner J, Ivison HE, Fuhr U, Arlt W (December 2010). "Impaired hepatic drug and steroid metabolism in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency". European Journal of Endocrinology. 163 (6): 919–24. doi:10.1530/EJE-10-0764. PMC 2977993. PMID 20844025.
- ^ Nicolo C, Flück CE, Mullis PE, Pandey AV (June 2010). "Restoration of mutant cytochrome P450 reductase activity by external flavin". Molecular and Cellular Endocrinology. 321 (2): 245–52. doi:10.1016/j.mce.2010.02.024. PMID 20188793. S2CID 29109570.
- ^ Sandee D, Morrissey K, Agrawal V, Tam HK, Kramer MA, Tracy TS, Giacomini KM, Miller WL (November 2010). "Effects of genetic variants of human P450 oxidoreductase on catalysis by CYP2D6 in vitro". Pharmacogenetics and Genomics. 20 (11): 677–86. doi:10.1097/FPC.0b013e32833f4f9b. PMC 5708132. PMID 20940534.
- ^ Agrawal V, Choi JH, Giacomini KM, Miller WL (October 2010). "Substrate-specific modulation of CYP3A4 activity by genetic variants of cytochrome P450 oxidoreductase". Pharmacogenetics and Genomics. 20 (10): 611–8. doi:10.1097/FPC.0b013e32833e0cb5. PMC 2940949. PMID 20697309.
- ^ Flück CE, Pandey AV (March 2016). "Impact on CYP19A1 activity by mutations in NADPH cytochrome P450 oxidoreductase". The Journal of Steroid Biochemistry and Molecular Biology. 165 (Pt A): 64–70. doi:10.1016/j.jsbmb.2016.03.031. PMID 27032764. S2CID 23498012.
- ^ Merla G, Howald C, Henrichsen CN, Lyle R, Wyss C, Zabot MT, Antonarakis SE, Reymond A (August 2006). "Submicroscopic deletion in patients with Williams-Beuren syndrome influences expression levels of the nonhemizygous flanking genes". American Journal of Human Genetics. 79 (2): 332–41. doi:10.1086/506371. PMC 1559497. PMID 16826523.
- ^ Charvat KA, Hornstein L, Oestreich AE (1991). "Radio-ulnar synostosis in Williams syndrome. A frequently associated anomaly". Pediatric Radiology. 21 (7): 508–10. doi:10.1007/bf02011725. PMID 1771116. S2CID 33765973.
- ^ Ichinose M, Tojo K, Nakamura K, Matsuda H, Tokudome G, Ohta M, Sakai S, Sakai O (June 1996). "Williams syndrome associated with chronic renal failure and various endocrinological abnormalities". Internal Medicine. 35 (6): 482–8. doi:10.2169/internalmedicine.35.482. PMID 8835601.
- ^ Partsch CJ, Pankau R, Blum WF, Gosch A, Wessel A (July 1994). "Hormonal regulation in children and adults with Williams-Beuren syndrome". American Journal of Medical Genetics. 51 (3): 251–7. doi:10.1002/ajmg.1320510316. PMID 8074154.
- ^ Wu L, Gu J, Cui H, Zhang QY, Behr M, Fang C, Weng Y, Kluetzman K, Swiatek PJ, Yang W, Kaminsky L, Ding X (January 2005). "Transgenic mice with a hypomorphic NADPH-cytochrome P450 reductase gene: effects on development, reproduction, and microsomal cytochrome P450". The Journal of Pharmacology and Experimental Therapeutics. 312 (1): 35–43. doi:10.1124/jpet.104.073353. PMID 15328377. S2CID 8292025.
External links
edit- Cytochrome+P450+Reductase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- GeneReviews/NCBI/NIH/UW entry on Cytochrome P450 Oxidoreductase Deficiency